
Numerous injuries and one death have been reported in connection with fractures to Boston Scientific’s now-recalled Emblem S-ICD device.
In December 2020, Boston Scientific and the U.S. Food and Drug Administration (FDA) coordinated a voluntary recall of nearly 20,000 Emblem S-ICD devices. The recall noted that the devices’ electrodes could fracture unexpectedly—meaning that the devices cannot provide life saving treatments.
ICDs, or implantable cardioverter defibrillators, help treat irregular heartbeats known as arrhythmias. If left untreated, arrhythmias can lead to serious problems such as sudden cardiac arrest, strokes, and heart failure. Since these complications can easily prove fatal, prompt treatment with an ICD can save lives. If these devices don’t work as intended, patients may be needlessly injured or killed.
According to the original recall, there were 26 injuries and one death reported in connection with device fracture. However, since the FDA’s recall announcement in December, numerous additional reports have been filed with the agency’s Manufacturer and User Facility Device Experience (MAUDE) Database. Many of these reports detail events from before the recall announcement, despite a more recent report date.
In some reports, including those which prompted the recall, doctors detail alerts triggered by electrode fracture. After this damage was identified, the devices were either removed, replaced, or left alone for further evaluation. For some lucky patients, this process did not involve any injuries or adverse events. Unfortunately, other patients were not as lucky.
A common issue detailed in reports is a rare pacemaker complication due in connection to device fracture. This complication, known as “Twiddler’s syndrome” occurs when intentional or unintentional movements from the patient result in the device becoming displaced. According to several MAUDE reports, Twiddler’s syndrome can occur with fractured Boston Scientific Emblem S-ICD electrodes.
Another common report is electric shocks to patients. One of the earliest reports of electric shock was from August 2019. According to this report, a patient received an electric shock from their device after falling on their chest. Although the report suspected that the detected device fracture stemmed from this fall, this incident may have been an early indicator of a larger problem.
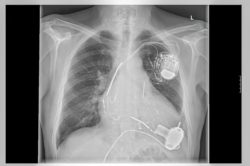
According to another report from November, one patient was exercising when they fainted and started to convulse. Although the device’s data didn’t identify a cardiac event behind these upsetting symptoms, the patient’s doctor supposedly suspects that electrode failure could be the source of the problem.
In addition to the aforementioned injuries connected to device failure, there has been one death reported with the Boston Scientific Emblem S-ICD. In February 2020, a patient died after their S-ICD emitted “high out of shock impedance measurements” and was prone to “oversensing of noise.” Although more information about the patient’s death is not available, the FDA MAUDE report notes that these defects were linked to fracture and resulted in a false positive signal in the device.
These reports detail events which may have been traumatizing and deeply upsetting to patients. In addition, these patients may have been forced to foot the bill for revision surgery or other medical treatment required to correct device fracture.
Although the emotional and financial consequences of device failure are significant, patients may have the ability to seek compensation, as Boston Scientific learned in a class action lawsuit filed by a group of Canadian women for complications with their transvaginal mesh product – leading to a $21.5 million settlement. By taking legal action, patients can aim to hold Boston Scientific accountable for their defective product.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2026 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Boston Scientific S-ICD Recall Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
Oops! We could not locate your form.













My husband passed 12/24/2017 as a result of a malfunctioning I’ve Fillter/defibulator