 The Food and Drug Administration (FDA) sent Pfizer a warning letter about the drug maker’s failure to conduct proper investigations into EpiPen malfunction reports.
The Food and Drug Administration (FDA) sent Pfizer a warning letter about the drug maker’s failure to conduct proper investigations into EpiPen malfunction reports.
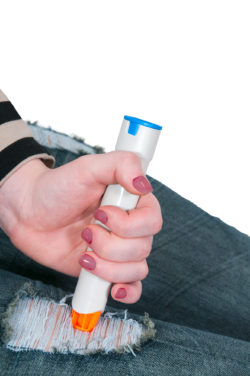
The EpiPen provides a potentially lifesaving dose of epinephrine to those experiencing severe allergic reactions, including anaphylaxis.
The device is supposed to fire and deliver the correct dose of medicine, but numerous complaints have been filed alleging that defective EpiPens failed to properly fire due to a defect in the component designed to deliver the drug.
Doctors often recommend that those with severe allergies keep EpiPens on hand in case of accidental exposure to an allergen. Additionally, school and other childcare centers also keep an EpiPen on hand in case a child has a severe reaction to an unknown allergy.
In its letter to Pfizer, the FDA states, “Our own data shows that you received hundreds of complaints that your EpiPen products failed to operate during life-threatening emergencies, including some situations in which patients subsequently died.”
The FDA warning letter indicates that the company failed to thoroughly investigate “multiple serious component and product failures” of EpiPen products and further failed to take action until the FDA launched an investigation.
According to the letter, the drug company had identified that certain EpiPens had a defect that caused them to fail to fire correctly. The letter notes that the lot was rejected and there was follow-up with the manufacturer, but the company allowed the manufacturer to continue to produce EpiPens with the same defective component.
“[W]e note that your follow up did not include removing potentially defective products from the marketplace, even though you had identified a defect in one of the critical components used to manufacture these products and even though you ultimately confirmed the same or similar component defect as the root cause for multiple complaints,” FDA’s warning letter states.
The defective EpiPens were manufactured by Meridian Medical Technologies, which is a unit of Pfizer. Meridian manufactures EpiPens for the drug company Mylan. According to the FDA warning letter, 13 lots of defective EpiPens have been recalled because of failure to operate. These recalls, noted the FDA, came after multiple discussions with the federal agency.
In addition to allegations of defective EpiPens, the lifesaving drug became a target of public scrutiny after the price shot up to $600 for a box of two. Only after being subject to congressional inquiry, did the company begin offering a generic option at a lower price.
The Department of Justice also targeted the drug maker, accusing the company of overcharging the federal government for the drug. The company recently announced it had settled those claims to the tune of $465 million.
A Pfizer spokesperson said in a statement that they were “very confident in the safety and efficacy of EpiPen products.” The Pfizer spokesperson also indicated the complaints may not be due to a defective EpiPen but because non-medically trained individuals often administer the drug.
“We currently have no information to indicate that there was any causal connection between these product complaints and any patient deaths,” noted Pfizer’s statement.
The FDA says that it received a number of “adverse event reports” related to defective EpiPens that failed to fire when administered. A spokesperson for the FDA noted that “an adverse event report does not establish a causal relationship between an adverse event and device failure, and reports do not always contain enough detail to properly evaluate an event.
Fill out the form on this page for a FREE case evaluation by the EpiPen attorneys at Product Safety Center.
In general, EpiPen lawsuits are filed individually by each plaintiff and are not class actions.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The EpiPen attorneys who work with Top Class Actions at the Product Safety Center will contact you if you qualify to let you know if an individual lawsuit or EpiPen class action lawsuit is best for you. Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2026 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free EpiPen Class Action Lawsuit Investigation
If you, or a loved one, experienced side effects from a defective EpiPen, you may have a legal claim. Submit your information now for a free case evaluation.
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
Please Note: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client, if you qualify, or getting you dropped as a client.
Oops! We could not locate your form.












