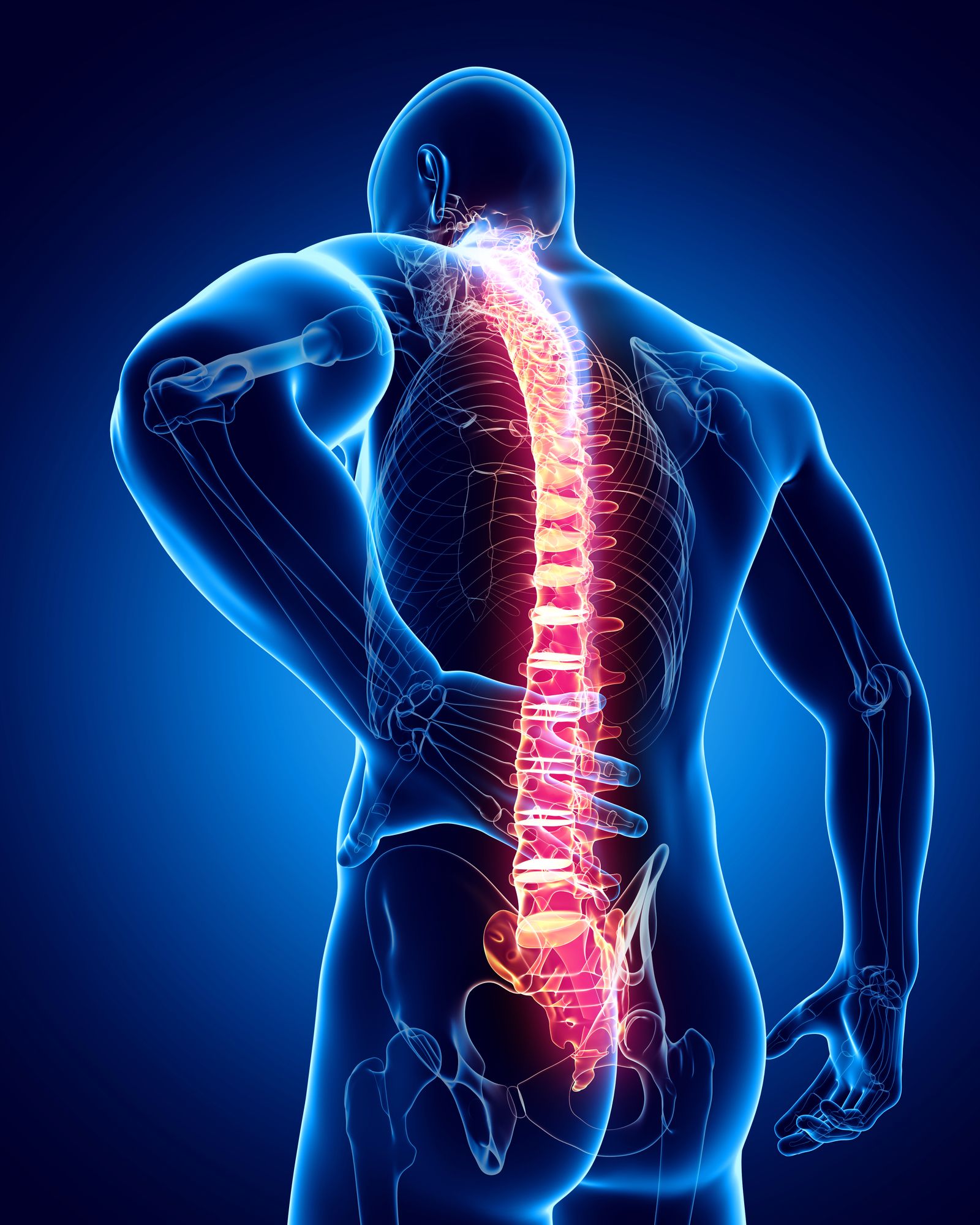
 Zimmer Biomet has issued a recall of two types of implantable SpF bone stimulator due to a serious risk of cytotoxicity.
Zimmer Biomet has issued a recall of two types of implantable SpF bone stimulator due to a serious risk of cytotoxicity.
The numerous Zimmer Biomet SpF bone stimulator devices that were recalled were thought to possess levels of harmful chemicals that are potentially toxic to organs and the body’s tissues.
According to the U.S. Food and Drug Administration, the agency announced that “Zimmer Biomet is recalling the SpF PLUS-Mini and SpF XL IIb Implantable Spinal Fusion Stimulators due to higher than allowed levels of potential harmful chemicals…” These increased levels that can be toxic to organs and tissues were reportedly found during Zimmer Biomet’s customary monitoring protocol.
According to the FDA, the cytotoxicity test conducted is a test that is part of the “biological evaluation of medical devices to ensure compatibility with the device and the human body.”
Moreover, the federal agency maintains that “a positive cytotoxicity test (failed result) can indicate that a device contains potential harmful chemicals at amounts or levels that could be dangerous to the patient.”
Any medical providers in possession of the affected medical device are instructed to quarantine the devices. Zimmer Biomet says it will schedule a time for removal from their medical facility by a Zimmer Biomet sales representative.
The serial numbers of the Zimmer Biomet SpF bone stimulator recalled includes products that were manufactured between Oct. 11, 2016 and Jan. 18, 2017 and distributed between March 28, 2017 and April 6, 2017. Thirty-three different serial numbers are identified as being affected by the recall.
According to the FDA, certain complications may result from use of the SpF bone stimulator. These include: chronic infections, paralysis, death, long- term hospitalization because of additional surgeries, and cytotoxicity.
Zimmer Biomet had started its recall of the SpF bone stimulator on April 20, 2017. It had been announced when the medical device maker had submitted to all its customers an Urgent Medical Device Removal notification.
How the SpF Bone Stimulator Works
The SpF bone stimulator is inserted into a patient’s back during spinal fusion surgery. The purpose of this procedure is to permanently fuse two or more bones located in the spine or vertebra. After the medical device is inserted, it applies constant electrical stimulation to the surgical area.
However, the manufacturer of the SpF bone stimulator discovered that the device contained higher than normal levels of cytotoxic chemicals that may harm organs and tissues in the body.
Ziimmer Biomet maintains that this discovery was made during the company’s customary monitoring protocol. They had also announced that the product may cause serious adverse side effects including: long-term hospitalization, cytotoxicity, long-term infections, death, and paralysis.
If you were the recipient of an SpF bone stimulator manufactured by Zimmer Biomet during your spine surgery, you may qualify for legal compensation. A free consultation with a knowledgeable attorney can help you understand more about your legal rights and opportunities to pursue legal relief.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or Zimmer Biomet class action lawsuit is best for you. [In general, Spinal Fusion Stimulator lawsuits are filed individually by each plaintiff and are not class actions.] Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2026 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Spinal Fusion Stimulator Class Action Lawsuit Investigation
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
Please Note: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client, if you qualify, or getting you dropped as a client.
E-mail any problems with this form to:
[email protected].
Oops! We could not locate your form.