 A Wisconsin man has filed a Stryker Accolade hip stem lawsuit, alleging that his failed hip replacement surgery caused severe complications including metal poisoning.
A Wisconsin man has filed a Stryker Accolade hip stem lawsuit, alleging that his failed hip replacement surgery caused severe complications including metal poisoning.
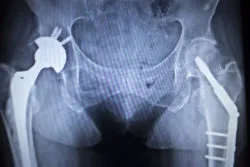
Plaintiff Alan K. underwent hip replacement surgery in July 2007 where he received the Stryker Accolade TMZF Hip Stem and LFIT Anatomic V40 Femoral Head implant.
He claims after the implantation of the hip stem that he suffered hip implant complications, that led to an additional revision surgery on March 2016.
During that surgery, Alan’s surgeon notes indicated “Neck trunnion has failed with metallosis of the hip due to titanium debris and instability of the femoral head component with impending dislocation.”
According to the Stryker Accolade hip stem lawsuit, as a direct result of the alleged defective devices, Alan has suffered and continues to suffer both injuries and damages including past, present, and future physical and mental pain and suffering; and past, present and future medical, hospital, rehabilitative and pharmaceutical expenses.
Alan also alleges Stryker knew or should have known of the risks of the metal hip implant but continued to aggressively market the device without adequate warnings, testing, or approval.
About the Stryker Accolade Hip Stem
On March 16, 2000, Stryker received FDA clearance to sell its Accolade prosthetic hip stem in the U.S. under the 510(k) process, claiming substantial similarity with other Howmedica Osteonics hip stems.
The Accolade hip implant featuring a titanium stem that is single piece designed to be implanted into the patient’s femur.
Although intended to provide improved flexibility and strength to mimic natural bone, some patients are alleging that the device poses a dangerous risk of corrosion and fretting.
The Accolade’s design is alleged to cause the release of metallic debris, such as chromium and cobalt particles, into a patient’s body. This may lead to localized tissue necrosis, bone damage, and metallosis or heavy metal poisoning.
Stryker Accolade Hip Implant Recalled
Stryker recalled their Accolade hip implant in July 2009. The U.S. Food and Drug Administration (FDA) announced that the Stryker Accolade hip implant constituted a class II recall.
The FDA defines a class II recall as “a situation in which use of or exposure to a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote.”
The FDA’s recall announcement noted that hip implants are inserted into the thigh bone to provide support to the femoral head in either an initial implantation procedure or a revision procedure.
Unlike most metal-on-metal hip implants, which are often made of cobalt and chromium, the Stryker Accolade hip implant is comprised of mostly titanium.
According to the FDA, Stryker recalled the device because specific lots of the device deviated from their specifications for “tensile bond strength and crystallinity”, which could lead to metal particles being released into a patient’s body.
Stryker has also had issues with its Rejuvenate and ABG II hip replacements, which were also recalled in 2012 amid reports that they corrode and fail within a few years of surgery.
Filing a Stryker Accolade Hip Stem Lawsuit
The Stryker Accolade hip stem has been linked to some patients suffering complications including:
- Infection
- Necrosis (premature tissue death)
- Metallosis
- Revision surgery
- Bone damage
- Pseudotumors
A Stryker Accolade hip stem lawsuit may be an option for patients who have suffered complications from the medical device.
Although these new type of hip implants use a proprietary titanium alloy aimed at easing the friction with bone surfaces, lawsuits have alleged that the design and manufacture of the hip implant is defective resulting in some patients suffering bone damage and heavy metal poisoning.
In fact, many lawsuits against the manufacturer have already been filed. These lawsuits specifically allege that the materials used in the Stryker Accolade hip stem have been known for decades to cause significant fretting and corrosion issues due to the combination of dissimilar metals.
Affected patients may be able to pursue claims for compensation with the help of an attorney. For more information about filing a Stryker Accolade hip stem lawsuit, contact an experienced defective hip implant attorney today for a free, confidential case evaluation.
The Stryker Accolade Hip Stem Lawsuit is Case No. 3:17-cv-00416-BRM-DEA filed in the U.S. District Court for the District of New Jersey.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The hip implant attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or class action lawsuit is best for you. [In general, metal hip implant lawsuits are filed individually by each plaintiff and are not class actions.] Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2025 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Stryker Metal Hip Replacement Class Action Lawsuit Investigation
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
Please Note: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client, if you qualify, or getting you dropped as a client.
Email any problems with this form to [email protected].
Oops! We could not locate your form.