 Tegretol (generic: Carbamazepine) is a widely-prescribed drug for the treatment of certain seizure disorders and bipolar disorders. Yet, this medication may cause serious Tegretol side effects, including Stevens Johnson Syndrome.
Tegretol (generic: Carbamazepine) is a widely-prescribed drug for the treatment of certain seizure disorders and bipolar disorders. Yet, this medication may cause serious Tegretol side effects, including Stevens Johnson Syndrome.
Like most prescription medications, Tegretol use may lead to side effects, some minor and some more severe that require hospitalization.
Common Tegretol side effects include: constipation or diarrhea, dizziness, drowsiness, dry mouth, headache, indigestion, itching skin or mild rash, nausea and vomiting.
However, Tegretol has been reported to be one of the most common causes of Stevens Johnson Syndrome, a life-threatening medication reaction that affects the mucous membranes and skin.
What is Stevens Johnson Syndrome?
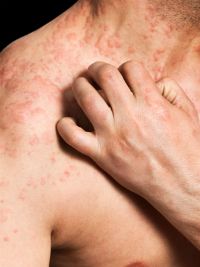
Stevens Johnson Syndrome (SJS) is a very rare, acute, serious, and potentially fatal skin reaction in which there is sheet-like skin and mucosal loss.
Stevens Johnson Syndrome usually develops within the first week of taking a medication or combination of medications but up to two months after starting the treatment therapy, though the mechanism has still not been understood and is complex.
But, for most drugs the onset of Stevens Johnson Syndrome is within a few days up to one month.
Before the signature rash appears, there is usually a illness of several days duration resembling an upper respiratory tract infection or flu-like symptoms. Initial Stevens Johnson symptoms may include:
- Fever
- Sore throat, difficulty swallowing
- Runny nose and cough
- Sore red eyes, conjunctivitis
- General aches and pains
There is then an abrupt onset of a tender, painful red skin rash and lesions starting on the trunk and extending rapidly over hours to days onto the face and limbs. Full-blown Stevens Johnson Syndrome is usually reached by four days.
These skin lesions may be flat, red and diffuse (measles-like spots) or purple spots or flaccid blisters. The blisters then merge to form sheets of skin detachment, exposing red, oozing dermis.
Patients with Stevens Johnson Syndrome are very ill and in considerable pain. In addition to skin and mucous membranes, other organs may be affected including liver, kidneys, lungs, bone marrow and joints.
Complications of Stevens Johnson Syndrome
Stevens Johnson Syndrome can be fatal due to complications in the acute phase. When the lesions and rash covers more than 30 percent of the body, the condition is upgraded to Toxic Epidermal Necrolysis (TEN).
The mortality rate is up to 10 percent for Stevens Johnson Syndrome and at least 30 percent for TEN.
During the acute phase, potentially fatal complications include:
- Dehydration and acute malnutrition
- Infection of skin, mucous membranes, lungs (pneumonia), septicaemia (blood poisoning)
- Acute respiratory distress syndrome
- Gastrointestinal ulceration, perforation and intussusception
- Shock and multiple organ failure including kidney failure
- Thromboembolism
Tegretol and Stevens Johnson Syndrome
Tegretol, the brand name for carbamazepine, was introduced to the market in 1968 under less than ideal circumstances. The U.S. Food and Drug Administration (FDA) initially refused to approve the drug, claiming that the product “would appear to be an agent that possesses significant toxicity.”
On April 10, 1967, following the drug manufacturer’s repeated attempts to introduce the drug to the U.S. market, the FDA approved Tegretol but only for the treatment of pain associated with trigeminal neuralgia, finding the medication had little to no margin of safety based on animal studies performed.
Five years later, Novartis Pharmaceuticals, began lobbying the FDA to approve Tegretol as a treatment for epilepsy, all the while hailing the drug’s safety and its non-carcinogenic nature.
The FDA was not impressed by the manufacturer’s alleged year-long animal study involving rats, which Novartis used to prove the drug to be non-carcinogenic. But the agency approved Tegretol as a drug of last resort, for use only with epileptic patients who did not respond to treatment with other drugs.
However, Novartis allegedly marketed Tegretol for use as a first-line treatment of epilepsy, plus for a broader use as an anticonvulsant drug and as a mood stabilizer.
In May 2010, the Madison-St. Clair Record reported that a group of 19 plaintiffs from across the country filed a class action lawsuit against Novartis claiming that doctors prescribed either them or their deceased relatives carbamazepine drug products.
The plaintiffs alleged that while marketing the drug’s efficacy, Novartis failed to inform the public of its associated dangers, such as Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Syndrome, both of which cause severe skin reactions, claims the Tegretol Stevens Johnson Syndrome lawsuit.
If you or a loved one have taken Tegretol (carbamazepine) and have been diagnosed with SJS or TENS or a loved one has died from complications Tegretol side effects including Stevens Johnson Syndrome, you should contact an experienced Tegretol attorney to discuss the legal options available to you and your family.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The Stevens Johnson Syndrome attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or class action lawsuit is best for you. [In general, SJS lawsuits are filed individually by each plaintiff and are not class actions.] Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2025 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Help for Victims of Stevens Johnson Syndrome
If you or a loved one were diagnosed with Stevens Johnson Syndrome (SJS) or toxic epidermal necrolysis (TEN) after taking a prescribed or over-the-counter medication, you may be eligible to take legal action against the drug’s manufacturer. Filing an SJS lawsuit or class action lawsuit may help you obtain compensation for medical bills, pain and suffering, and other damages. Obtain a free and confidential review of your case by filling out the form below.
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
Please Note: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client, if you qualify, or getting you dropped as a client.
Oops! We could not locate your form.












I was given tegratol as a child for seizure an I had the sjs rash an it messed up my kidneys an now I have cysts in my right kidney iam 40 years old now this happened when I was 4 years old I want to know what can be don’t for all the pain I have had all of these years an now to this day my kidneys hurt so bad