 As new diabetes drugs spend more time on the market, scientists are gradually becoming more familiar with their longer-term side effects.
As new diabetes drugs spend more time on the market, scientists are gradually becoming more familiar with their longer-term side effects.
A recent announcement by the FDA addresses some potentially dangerous side effects of Invokanaand other diabetes drugs in its class.
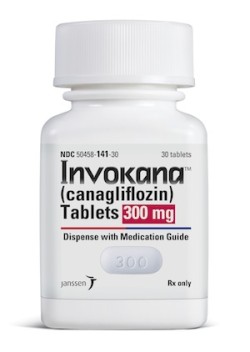
Invokana is approved by the FDA as a treatment for the high blood sugar associated with type 2 diabetes, to be used in conjunction with exercise and restrictions on diet.
It’s also known by its generic name canagliflozin. It’s approved by the FDA to treat type 2 diabetes but not type 1.
The drug is in a class of diabetes medications called SGLT2 inhibitors. These drugs lower blood sugar by stimulating the kidneys to remove more of it from the blood so it can be excreted in the urine.
FDA Warns About Invokana Side Effects
Invokana’s label was updated in December 2015 to include warnings about ketoacidosis and urinary tract infections leading to possible kidney infections as possible Invokana side effects.
In announcing the new warning, the FDA said that a review of its Adverse Event Report database had revealed 73 cases of ketoacidosis from March 2013 to May 2015 in patients taking SGLT2 inhibitors like Invokana.
Ketoacidosis is a seriously dangerous condition in which the blood becomes too acidic.
The FDA says that persons taking an SGLT2 inhibitor like Invokana should stop taking it and seek medical attention immediately if they experience any symptoms of ketoacidosis.
According to the FDA’s announcement, symptoms of ketoacidosis include “nausea, vomiting, abdominal pain, tiredness, and trouble breathing.”
Other symptoms can include a fruity smell on the breath, caused by the higher levels of ketones in the blood.
The ketoacidosis that has been reported in Invokana patients is different from the kind that’s normally associated with diabetes. Diabetic ketoacidosis occurs much more commonly in patients with type 1 diabetes, which Invokana is not designed to treat.
The lack of insulin caused by type 1 diabetes prevents cells from metabolizing sugar to get energy, so they metabolize fat instead. Fat metabolism produces acidic compounds called ketones. These ketones can build up in the bloodstream, raising its acidity and eventually leading to ketoacidosis.
The sugar that the cells fail to metabolize remains in the blood. For that reason, elevated blood sugar levels can be a telltale sign of diabetic ketoacidosis.
Invokana ketoacidosis, on the other hand, does not usually co-occur with high blood sugar.
The FDA notes that some cases of ketoacidosis reported in conjunction with Invokana therapy were not recognized right away as ketoacidosis, because they did not feature the expected increase in blood sugar.
Other Invokana Side Effects
The new FDA warning also advises consumers about the possibility of urinary tract infections that in some cases have led to kidney failure.
A review of the agency’s Adverse Event Reporting System revealed 19 cases of urinary tract infections that progressed into either life-threatening blood infections or kidney infections.
Some of these patients suffered from kidney failure that required dialysis or intensive care treatment.
In general, Invokana lawsuits are filed individually by each plaintiff and are not class actions.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or class action lawsuit is best for you. Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2025 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Invokana Class Action Lawsuit Investigation
If you or a loved one suffered ketoacidosis or kidney failure after taking Invokana, you may have a legal claim. See if you qualify to pursue compensation and join a free diabetes medication class action lawsuit investigation by submitting your information for a free case evaluation.
An attorney will contact you if you qualify to discuss the details of your potential case.
Oops! We could not locate your form.