 DePuy Orthopaedics and parent company Johnson & Johnson is facing a growing multidistrict litigation (MDL), consisting of product liability claims regarding the Pinnacle Acetabular Cup System.
DePuy Orthopaedics and parent company Johnson & Johnson is facing a growing multidistrict litigation (MDL), consisting of product liability claims regarding the Pinnacle Acetabular Cup System.
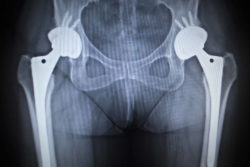
The Pinnacle Acetabular Cup System is a metal on metal hip replacement system, which is typically recommended to patients suffering from bone deterioration in the hips and consists of artificial ball and socket joint components.
The Pinnacle Acetabular Cup System is supposed to provide support and movement for patients suffering from hip arthritis or other problems but has been associated with serious metal hip implant complications.
This was allegedly the case with one of the most recent product liability claims to join the MDL, which comes from a woman from California who alleges the manufacturing company failed to warn her against the allegedly defective nature of the Pinnacle Acetabular Cup System.
Plaintiff Virginia K. says she was recommended the Pinnacle Acetabular Cup System after her physician had reviewed the marketing materials provided by DePuy, which stated the prosthetic was safe and reliable for patient use. With these benefits in mind, Virginia underwent hip replacement surgery on her right side on April 21, 2016.
However, not long after the Acetabular Cup System was implanted, Virginia had reportedly begun experiencing painful side effects. Virginia reportedly experienced symptoms often associated with metal on metal hip replacement failure, including elevated metal ion levels in the blood. This can result in a number of serious complications including:
- Metallosis (Blood Metal Poisoning)
- Bone Erosion
- Tissue Death
- Debilitating Pain
- Fretting
- Corrosion
- Formation of Pseudotumors
According to the DePuy Pinnacle lawsuit, Virginia became aware of a potential causal link between her injuries and the Acetabular Cup System in July 2016. Not long after her discovery, Virginia decided to file legal action against the company for allegedly failing to warn her against possible metal hip implant complications.
Overview of DePuy Pinnacle Complications
The DePuy Pinnacle Acetabular Cup System was approved by the FDA in 2000 and has since been the subject of numerous hip replacement failure report. Like other metal on metal hip implant systems, the DePuy Pinnacle Acetabular Cup System was soon associated with the same problems as earlier metal on metal hip implants.
According to Virginia’s DePuy Pinnacle lawsuit, there have been over 1,300 injury reports submitted to the FDA regarding the metal on metal hip implant system. When all-metal hip implants were first approved to enter the market, they were thought to be overtly superior to plastic or ceramic models.
However, it was soon discovered that the all metal ball and socket joint component could shed metal ions into the bloodstream, which can cause a number of other problems. Virginia states that she would not have agreed to have the Acetabular Cup System if she had known about the defective nature of the device.
Virginia’s DePuy Pinnacle lawsuit is joining MDL No. 2244, where it will stand alongside other claims alleging problems with the Pinnacle Acetabular Cup System. By joining an MDL, Virginia’s claim will be streamlined through the litigation process and will avoid potential problems like conflicting rulings from different judges.
This DePuy Pinnacle Lawsuit is Case No. 3:18-cv-01359-K, in the U.S. District Court of Northern Texas, Dallas Division.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The hip implant attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or class action lawsuit is best for you. [In general, metal hip implant lawsuits are filed individually by each plaintiff and are not class actions.] Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2026 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free DePuy, Zimmer Hip Replacement Lawsuit Investigation
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
[email protected].
Oops! We could not locate your form.













2 thoughts onPinnacle Acetabular Cup System Allegedly Causes Bone Deterioration in Patient