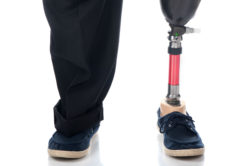
 A new Invokana lawsuit is being filed against Johnson & Johnson and the company’s subsidiary, Janssen Pharmaceuticals by a man alleging that he suffered severe adverse drug side effects and complications including a below knee amputation.
A new Invokana lawsuit is being filed against Johnson & Johnson and the company’s subsidiary, Janssen Pharmaceuticals by a man alleging that he suffered severe adverse drug side effects and complications including a below knee amputation.
The plaintiff, Keener B., a resident and citizen of Pensacola, Fla., filed the Invokana lawsuit in New Jersey federal court joining a growing multidistrict litigation (MDL) against the companies.
Keener says that he began taking Invokana (canagliflozin) to treat diabetes and minimize and control his blood sugar level in August 2014. However, he says that Invokana caused him to suffer severe injuries including “heart attack, stroke, renal failure, renal impairment, renal insufficiency, kidney injury, diabetic ketoacidosis and amputations,” the Invokana lawsuit states. He claims his below knee amputation was one of the most severe side effects.
On June 5, 2017, Keener says that he suffered a below knee amputation of the right leg. The injury required substantial medical treatment. Keener claims that due the companies’ lack of proper safety studies and “failure to properly assess and publicize alarming safety signals” that he suffered these complications.
Keener says that had he known of the risks associated with Invokana, he would have never ingested the medication.
The Invokana lawsuit was filed on June 5, 2018, raising multiple claims including strict liability, manufacturing defect, design defect, failure to warn, negligence, breach of express warranty, breach of implied warranty, negligent misrepresentation, fraudulent misrepresentation, unjust enrichment, fraud, violation of consumer protection laws, punitive damages.
Keener demands a trial by jury.
Invokana Lawsuit Joins MDL
Keener’s claim is filed as part of a large multidistrict litigation (MDL) centered on allegations that Invokana (canagliflozin) causes serious complications including diabetic ketoacidosis and below knee amputation.
An Invokana black box warning was added to type-2 diabetes medications, Invokana, Invokamet, and Invokamet XR, by the U.S. Food and Drug Administration (FDA) warning of below knee amputation risks.
According to the May 2017 announcement they stated that, “based on new data from two large clinical trials, the U.S. Food and Drug Administration (FDA) has concluded that the type 2 diabetes medicine canagliflozin (Invokana, Invokamet, Invokamet XR) causes an increased risk of leg and foot amputations.”
“We are requiring new warnings, including our most prominent Boxed Warning, to be added to the canagliflozin drug labels to describe this risk.”
Adverse side effects that have also been linked to Invokana have included:
- Amputation Risks
- Diabetic Ketoacidosis (DKA)
- Lactic Acidosis
- Kidney and Blood Infections
- Acute Kidney Injury Warning
Invokana became approved by the FDA on March 29, 2013, to be taken in combination of diet and exercise. It belongs to a class of medications termed sodium-glucose contransporter-2 (SGLT2) inhibitors.
The FDA ordered black box warning in May 2017, is the highest warning the FDA may issue. The warning was based off two clinical trials that discovered that patients prescribed Invokana were twice as likely to suffer from an amputation than patients that had taken a placebo.
The Invokana Lawsuit is Case No. 3:18-cv-10187-BRM-LHG, in the U.S. District Court for the District of New Jersey. The Invokana Lawsuit is MDL No. 2750 is in re: Invokana (Canagliflozin) Products Liability Litigation, in the U.S. District Court for the District of New Jersey.
In general, Invokana and Invokamet lawsuits are filed individually by each plaintiff and are not class actions.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or class action lawsuit is best for you. Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2025 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Invokana Class Action Lawsuit Investigation
If you or a loved one suffered ketoacidosis or lower extremity amputation after taking Invokana, Invokamet, or Invokamet XR, you may have a legal claim. See if you qualify to pursue compensation and join a free diabetes medication class action lawsuit investigation by submitting your information for a free case evaluation.
An attorney will contact you if you qualify to discuss the details of your potential case.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
Oops! We could not locate your form.












