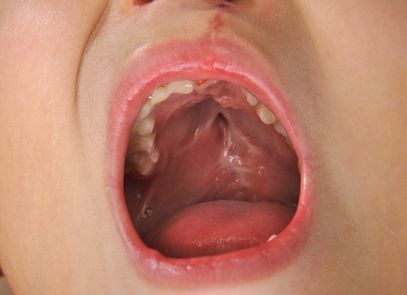
 A mother’s use of the popular anti-nausea drug Zofran caused her child to be born with cleft lip and cleft palate congenital malformations, according to a new Zofran birth defect lawsuit.
A mother’s use of the popular anti-nausea drug Zofran caused her child to be born with cleft lip and cleft palate congenital malformations, according to a new Zofran birth defect lawsuit.
Plaintiff Maria A. filed the Zofran lawsuit on behalf of herself and her daughter, who is identified in the complaint as G.A.. The baby girl was born in October 2012 with cleft lip and cleft palate birth defects after being exposed to Zofran during pregnancy.
Cleft palate is a birth defect where the roof of the baby’s mouth fails to properly develop and close. Cleft lip is a birth defect where the baby is left with a split in the upper lip. Babies born with a cleft lip or cleft palate due to exposure to Zofran during pregnancy often require surgery during the first year of life and may be still contend with health problems going forward.
According to court documents, GlaxoSmithKline (GSK) has also been accused of illegally promoting Zofran off-label as a treatment for morning sickness without ever conducting any studies to determine that the anti-nausea drug was safe for pregnant women and their unborn babies.
“GSK avoided conducting these studies and buried any internal analyses of Zofran’s teratogenic potential because they would have hampered its marketing of Zofran and decreased profits by linking the drug to serious birth defects,” the Zofran lawsuit claims. “GSK’s conduct was tantamount to using expectant mothers and their unborn children as human guinea pigs.”
Maria’s Zofran lawsuit joins a growing number of complaints brought against GSK in recent years. These Zofran lawsuits are generally filed on behalf of families across the country who believe that their children have suffered serious birth defects from Zofran.
What is Zofran?
Zofran (ondansetron) is a widely used anti-nausea drug commonly prescribed off-label to pregnant women suffering from severe morning sickness. It is important to note that the drug has never been approved by the U.S. Food and Drug Administration (FDA) for this particular use.
GSK promoted the prescription of Zofran for morning sickness, despite not being approved for such, because this tactic expanded the market for this drug and proved to be extremely profitable for the drug maker.
Unfortunately for GSK, a number of studies have been published recently indicating that women who take Zofran during the first trimester of pregnancy may have a significantly increased risk of giving birth to babies with devastating birth defects, including cleft lip and cleft palate.
Zofran Birth Defect Studies
The potential link between Zofran and birth defects became evident as early as 2006, when researchers from Hong Kong published a study demonstrating that Zofran crosses the placenta in significant amounts when taken by pregnant women.
Another study, published in the November 2011 edition of Birth Defects Research Part A: Clinical and Molecular Teratology, concluded that babies exposed to Zofran in utero may be 2.37 times more likely to develop cleft palate birth defects, most likely caused by an interruption in the development of the fetus in early pregnancy.
Additionally, a control study published in the medical journal Birth Defects Research Part A indicated a link between Zofran and cleft palate. The study concluded that women prescribed the medication for morning sickness during the first trimester of pregnancy may be 2.3 times more likely to give birth to a child with this facial defect.
Despite these findings, the U.S. Food and Drug Administration recently rejected a petition to change Zofran’s drug category, saying in an October 2015 statement that these studies “do not support a determination that there is an increased risk of fetal adverse outcomes.”
Zofran Lawsuits
As of August 2015, there were at least 33 Zofran lawsuits filed in 20 different federal district courts on behalf of families of children who were born with severe birth defects after allegedly being exposed to Zofran in utero.
If you believe your child has been adversely affected by birth defects from Zofran use in pregnancy, you may be entitled to financial compensation for your child’s injuries, medical expenses and emotional trauma, which you can pursue by filing a Zofran claim against GlaxoSmithKline.
The Zofran Birth Defect Lawsuit is Case No. 5:15-cv-01780, in the U.S. District Court of the Central District of California.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The birth defect attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual Zofran lawsuit or Zofran class action lawsuit is best for you. [In general, Zofran lawsuits are filed individually by each plaintiff and are not class actions.] Hurry — statutes of limitations may apply.
Get Help – It’s Free
Join a Free Zofran Birth Defects Class Action Lawsuit Investigation
If you or someone you know took Zofran while pregnant and had a baby with a birth defect, you or this person may have a legal claim. See if you qualify by filling out the short form below.
A Zofran birth defect attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
Oops! We could not locate your form.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2025 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.












