 In December 2013, the U.S. Food and Drug Administration issued a Drug Safety Communication for the drug Clobazam (also sold under the brand name Onfi). Clobazam is an anticonvulsant designed to treat certain classes of seizures. The Drug Safety communication advised that the drug was linked to a “rare but serious” autoimmune reactions including Stevens Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN).
In December 2013, the U.S. Food and Drug Administration issued a Drug Safety Communication for the drug Clobazam (also sold under the brand name Onfi). Clobazam is an anticonvulsant designed to treat certain classes of seizures. The Drug Safety communication advised that the drug was linked to a “rare but serious” autoimmune reactions including Stevens Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN).
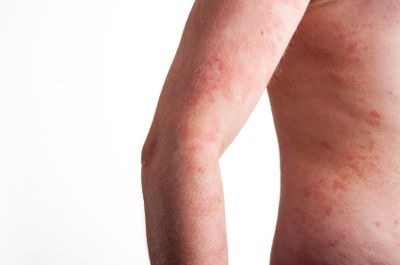
An autoimmune disease is a disease wherein the body attacks its own tissue, mistaking it for invading disease organisms. In SJS and TEN, the body attacks its own skin. SJS starts with flu-like symptoms that progresses to a painful skin rash with ulcers and blisters. It may also damage the patient’s eyes and internal organs. The most serious cases of SJS escalate to TEN, where large patches of the skin die and slough off, leaving burn-like wounds. Both conditions can be fatal and leave the patient with permanent disability and disfigurement.
The Drug Safety Communication indicated that the FDA has identified twenty cases of SJS/TEN via its postmarket surveillance apparatus. As per federal law, reports of complications are compiled and analyzed by the agency. The postmarket surveillance also listed two deaths as being possibly linked to the seizure drug.
Sometimes, serious risks like SJS/TEN may not become obvious until the drug hits the market. Even the largest-scale trials may not include enough test subjects to reveal less-common side effects like SJS and TEN. Thus, the link between drugs like Clobazam and SJS/TEN cannot be demonstrated until the drug hits widespread use among the public. In cases like these, the FDA may mandate changes to a drug’s warning label or through Drug Safety Communications.
The announcement by the FDA does not effect the drug’s approval. The FDA does advise patients to urgently seek medical attention if they experience a rash after starting treatment with Clobazam. The sudden discontinuation of seizure medicine can have serious consequences of its own, so the FDA advises consulting a physician as soon as possible if a patient begins to show signs of SJS or TEN.
A class action lawsuit investigation has been launched to explore the possibility of legal action against the manufacturers of drugs like Clobazam that have been linked to complications like SJS and TEN. Such a lawsuit could allege that manufacturers are aware of these risks, and failed to warn the public in a timely manner. Such a suit could seek damages related to emergency medical care, loss of income potential, and wrongful death connected with SJS and TEN.
In general, SJS lawsuits are filed individually by each plaintiff and are not class actions.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or class action lawsuit is best for you. Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2026 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Help for Victims of Stevens Johnson Syndrome
If you or a loved one were diagnosed with Stevens Johnson Syndrome (SJS) or toxic epidermal necrolysis (TEN) after taking a prescribed or over-the-counter medication, you may be eligible to take legal action against the drug’s manufacturer. Filing an SJS lawsuit or class action lawsuit may help you obtain compensation for medical bills, pain and suffering, and other damages. Obtain a free and confidential review of your case by filling out the form below.
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
Oops! We could not locate your form.












