 A late diabetes patient’s family claims she suffered injury from saxagliptin heart failure.
A late diabetes patient’s family claims she suffered injury from saxagliptin heart failure.
Plaintiff Audrey G., serving individually and as the administrator of the estate of Ophelia D., claims that Bristol-Meyers Squibb Company, AstraZeneca Pharmaceuticals LP, and McKesson Corporation concealed serious side effects associated with their diabetes drugs, Obglyza and Kombiglyze XR. Audrey claims that the use of one or both of these drugs caused serious injury to the late Ophelia.
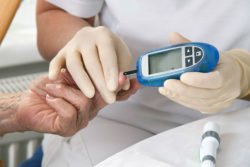
Onglyza and Kombiglyze XR are brand-name drugs used to treat type-2 diabetes. The active drug in these two medications is saxagliptin. Saxagliptin is used to help lower blood sugar in patients with type-2 diabetes, but has been linked to an increased risk of heart failure. This can cause serious injury and death, according to Audrey G.’s saxagliptin heart failure lawsuit.
Allegedly, Bristol-Meyers Squibb and the other manufacturers of the Onglyza and Kombiglyze XR failed to warn Ophelia’s physicians of the serious side effects that could result from taking the drugs. Audrey claims that Ophelia suffered severe physical, emotional, and financial injury from taking these drugs. Allegedly, Ophelia suffered cardiac failure after taking Saxagliptin.
The saxagliptin heart failure lawsuit states that saxagliptin is a DPP4 inhibitor, meaning that it lowers blood sugar by inhibiting natural enzymes from stopping the GLP-1, an amino acid hormone that regulates the production of insulin. So, this allows the body to produce insulin for longer than a body normally would after a meal, thus lowering blood sugar in people with type-2 diabetes.
The saxagliptin heart failure lawsuit goes on to note that in an unmedicated person, GLP-1’s half-life, or the amount of time it stays in a person’s system, is only two minutes, whereas in a person taking saxagliptin, GLP-1 remains in a person’s system for nearly three hours. Audrey claims that the drug companies failed to sufficiently investigate the effects on this enzyme and hormone change on a patient’s entire system, thereby ignoring possible side effects while declaring the drug effective in lowering blood sugar.
According to Audrey, the drug companies should have tested to see if this systemic hormone change would have an impact on a patient’s cardiovascular health. Allegedly, patients with type-2 diabetes already have an elevated risk of cardiac issues, so to make sure a drug is safe for diabetes patients, the companies should have tested to see if the drugs affected cardiac health.
In support of this argument, Audrey notes that in 2008, the FDA issued guidelines to drug manufacturers developing drugs for diabetes, stating that “new anti-diabetic medications for the treatment of type-2 diabetes should demonstrate their products are not associated with an unacceptable increase in cardiovascular risk.” According to Audrey, this information was available when the companies were testing Onglyza and Kombiglyze XR, which were then released in 2009 and 2010, respectively, yet they failed to perform tests in adherence with the FDA’s guidelines.
Allegedly, Bristol-Meyers Squibb and the other manufacturers knowingly marketed the drug while having insufficiently studied the effects of the drugs, and failed to warn medical professionals and patients like Ophelia about the potential risks of the drug.
The Saxagliptin Heart Failure Lawsuit is Case No. 5:18-md-02809-KKC, in the U.S. District Court for the Eastern District of Kentucky, Central Division — Lexington.
Free Onglyza Lawsuit or Kombiglyze Lawsuit Review
Did you or a loved one suffer heart failure, cardiac failure, congestive heart failure or death after taking Onglyza or Kombiglyze XR? If so, you may be eligible to join a FREE Onglyza lawsuit and Kombiglyze lawsuit investigation and pursue compensation for your injuries. Fill out the form on this page to see if you qualify!
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2025 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Onglyza Lawsuit or Kombiglyze XR Lawsuit Investigation
If you have been injured or if you lost a loved one due to Onglyza side effects or Kombiglyze XR side effects such as heart failure, cardiac failure, or congestive heart failure, you may have a legal claim. See if you qualify to pursue compensation and join a free Onglyza or Kombiglyze XR investigation by submitting your information for a free case evaluation.
An attorney will contact you if you qualify to discuss the details of your potential case.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
Oops! We could not locate your form.