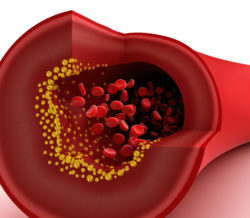
The CML drug Tasigna was connected to atherosclerosis—a condition where arteries become hardened by a build-up of fatty deposits. Depending upon their location, these fatty deposits can contribute to serious health situations such as disability or sudden death from stroke as well as heart or respiratory arrest.
These consequences are often brought on by a combination of plaque breaking off and blocking systemic blood transport and by the reduced diameter in the involved artery.
The CML drug Tasigna is manufactured and distributed by the Novartis Co. Novartis got approval from the U.S. Food and Drug Administration in 2007 to commercially market the medication domestically.
It works to bring this particular cancer under control by blocking bodily production of a type of protein which would otherwise feed the disease by allowing cancerous cells to grow and multiply.
The protein which is blocked by the CML drug Tasigna is called BCR-AB1. When this process is effectual, continued advancement of this leukemia is seen to slow and often stop leading to remission of the patient.
While Novartis has alerted consumers of an issue through a “black box” warning system that can affect heart rhythm and cause sudden myocardial infarction or heart attack, the company has never mentioned any concern regarding the development of partial or complete arterial blockages while taking the CML drug Tasigna.
Complainants that have filed respective lawsuits against the Novartis Co. for their failure to warn of this serious and unexpected side effect claim that the medication brought on serious atherosclerosis within a short window of taking the CML drug Tasigna.
By the time the medical issue was discovered in many recipients, the circulatory problem was so advanced that it was determined there was little recourse to bring it under control.
Plaintiffs filing cases against Novartis have also noted that a great deal of time has elapsed since the company first made a label change alerting Canadian consumers of the threat of “hardening of the arteries” secondary to taking the cancer treatment medication. This was done in 2013, while no corresponding effort was made to warn American consumers of this potential adverse event.
If you or a loved one have taken Tasigna for CML and experienced severe cardiovascular issues related to PAD or arteriosclerosis, you may have a legal claim.
In general, Tasigna lawsuits are filed individually by each plaintiff and are not class actions.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual Tasigna lawsuit or Tasigna class action lawsuit is best for you. Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Tasigna Lawsuit Investigation
If you suffered from a serious side effect or a loved one died while taking Tasigna, you may have a legal claim. See if you qualify to pursue compensation and join a free Tasigna lawsuit investigation by submitting your information for a free case evaluation.
An attorney will contact you if you qualify to discuss the details of your potential case.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
Oops! We could not locate your form.












