 The multiple sclerosis (MS) drug Zinbryta was voluntarily withdrawn from the worldwide marketplace because of adverse event reports linking it to autoimmune encephalitis—a condition in which the body’s immune system starts attacking healthy brain cells.
The multiple sclerosis (MS) drug Zinbryta was voluntarily withdrawn from the worldwide marketplace because of adverse event reports linking it to autoimmune encephalitis—a condition in which the body’s immune system starts attacking healthy brain cells.
Biogen and AbbVie, the medication’s manufacturers, didn’t wait for the U.S. Food and Drug Administration (FDA) to force their hand, although only three cases of autoimmune encephalitis have been reported domestically.
As reported, most of the other autoimmune encephalitis cases have been seen in Europe and elsewhere. Since Zinbryta’s May 2016 FDA approval, however, the drug oversight agency has had an awareness of what they refer to as the drug’s “complex safety profile.”
Because of this, physicians were encouraged to only offer it to MS patients whose condition has not improved on at least two other medication regimens.
In addition to autoimmune encephalitis, there have been side effects purportedly linked to Zinbryta that have been registered in 1,200 different negative complaints passed on to the FDA’s Adverse Event Reporting System (FAERS). While only 25 of these situations resulted in the death of the patient, a full 626 of the reports involved serious health threats.
There was a full black box warning added to the label of Zinbryta after it became commercially available concerning liver and other potential issues resulting from its use. This warning mentioned the risk of liver injury and subsequent possibility of organ failure. It warned not to prescribe the drug to patients with a prior history of hepatic compromise.
The warning also mentioned “immune-mediated” disorders, generally referring to mild skin rashes up to and including Stevens Johnson Syndrome, an allergic reaction to some medications that can result in widespread damage or “burning” of the skin which can leave the body open to serious, life-threatening infection.
Despite the adding of this label, no move was made by Biogen and Abbvie to discontinue the medication—a very rare action on the part of a pharmaceutical company—until the European Medicines Agency (EMA) started their investigation of Zinbryta’s potential role in the development of a dozen cases of autoimmune encephalitis and meningoencephalitis.
What is Autoimmune Encephalitis?
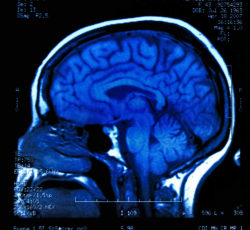
Autoimmune encephalitis is brought on by the patient’s own body treating its own nervous system tissue—brain cells—as if they were an invading foreign body. The result is overall inflammation of the brain inside the skull cavity which can be extremely dangerous and life-threatening.
Nervous system and/or psychiatric symptoms of autoimmune encephalitis can be varied and include but not be limited to:
- Balance or gait impairment
- Memory impairment
- Speech impairment
- Brain fog or difficulty processing information
- Auditory or visual hallucinations
- Panic attacks
- Aggressive acting out
- Acting out with sexually-inappropriate behaviors
- Seizures, fainting, and/or coma
The FDA Continues Probe
According to Marketwatch, the FDA is using all global data in its review of Zinbryta. At the same time, it is assisting the pharmaceutical company with an organized withdrawal of the drug from the U.S. market which should have been completed by April 30, 2018.
If you or a loved one were taking Zinbryta for MS and experienced any symptoms of autoimmune encephalitis, you may be eligible to join a class action lawsuit investigation that could regain you monies for medical care and hospitalization as well as pain and suffering.
Join a Free Zinbryta Class Action Lawsuit Investigation
If you or a loved one were diagnosed with encephalitis, liver injury, Stevens Johnson Syndrome (SJS), Toxic Epidermal Necrolysis (TEN), or another complication after taking Zinbryta, you may have a legal claim. Filing a Zinbryta lawsuit or joining this Zinbryta class action lawsuit investigation could help you recover compensation for medical bills, pain and suffering, and more.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2025 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Zinbryta Class Action Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
[email protected].
Oops! We could not locate your form.