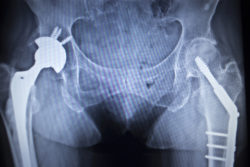
 A woman has filed suit alleging her Accolade TMZF hip stem failed due to a defectively implanted Stryker LFIT V40 femoral head.
A woman has filed suit alleging her Accolade TMZF hip stem failed due to a defectively implanted Stryker LFIT V40 femoral head.
Plaintiff Jeannette Z. had operations on both hips. She received the Accolade TMZF hip stem and the LFIT V40 femoral head on her right hip on Aug. 17, 2006. After tremendous pain and implant failure, she says, she eventually had the femoral head taken out on July 21, 2017.
On April 26, 2007, Jeannette was implanted with the same parts on her left hip. She says she has not yet scheduled surgery for the removal of the femoral head on her left hip.
Accolade TMZF Hip Stem Lawsuit Includes Metallosis Claims
Both the stem and head are made of different metals that scrape against each other when fitted in the hip. The Stryker LFIT V40 femoral head is a modular ball, consisting of a cobalt and chromium alloy. Stryker marketed the V40 head and the TMZF stem to be used together to produce better hip mobility and a lowered risk of dislocation.
However, doctors who have studied the failed parts say that Stryker should not have sold the Accolade TMZF hip stem in conjunction with the LFIT V40 femoral head at all. They claim the Accolade TMZF hip stem should have been marketed only with ceramic heads.
When the LFIT V40 femoral heads are paired with the Accolade TMZF hip stems, the result is friction between the two metal alloys that may cause metal particles to fret away. These particles work their way into surrounding tissue, which reacts by increasing the inflammatory response and retaining fluid as the body tries to expel these tiny metal invaders. As a result, the surrounding tissue may die and metallic toxins may leach into the bones and enter the blood stream.
Patients such as Jeannette who have received the combination of the LFIT V40 femoral head and Accolade TMZF hip stem can undergo an easy blood test to evaluate the metals in the blood. Depending upon the results, the blood test could determine if metallosis has begun and if the device has deteriorated.
Unfortunately, those experiencing toxic levels of chromium and cobalt in their blood may not be aware that their hips are corroding and that they are slowly being poisoned.
In July 2012, the journal Orthopedics published a study that determined nearly 95 percent of patients who had metal-on-metal hip implant systems had to undergo revision surgery within three years because of various failures in those systems.
Any hip implant patient experiencing impaired mobility, chronic pain, inflammation, implant loosening or dislocation should seek medical attention to determine of a mismatch of an LFIT V40 head and Accolade TMZF hip stem is the problem.
The Accolade TMZF Hip Stem Lawsuit is Case No. 1:17-cv-12449-IT and is part of the Stryker MDL, In re: Stryker LFIT V40 Femoral Head Products Liability Litigation, MDL No. 17-md-2768-IT in the U.S. District Court for the District of Massachusetts.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The hip implant attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or class action lawsuit is best for you. [In general, metal hip implant lawsuits are filed individually by each plaintiff and are not class actions.] Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2025 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Stryker Metal Hip Replacement Class Action Lawsuit Investigation
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
Please Note: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client, if you qualify, or getting you dropped as a client.
Email any problems with this form to [email protected].
Oops! We could not locate your form.