Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
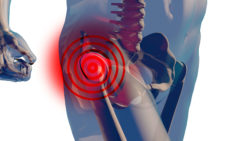
Prior to this event, the LFIT Anatomic Cobalt-Chromium V40 femoral head had been the subject of numerous complaints directed toward the Stryker Co. The complaints indicated that patients had experienced adverse effects from alleged taper lock failures of the hip prosthetic.
The LFIT V40 femoral head is attached to a stem implanted in the femur. While the stem is supposed to stabilize the ball of the joint which rotates in the acetabular cup in the pelvic cage, this stem had been implicated in multiple reports of a loosening of the head or even complete disassociation. According to the FDA’s recall announcement, the Stryker femoral head recall affected 42,519 distributed units.
The Stryker Howmedica Osteonics Corp. sent out notice of the Stryker femoral head recall the end of August to distributing branches and agents asking them to quarantine any LFIT V40’s that they had in stock as prevention from selling and implanting a defective product into an unknowing and unsuspecting patient. Notification was also sent on that date to appropriate hospital risk management departments and associated orthopedic surgeons.
By early October 2016, the Stryker Co. had identified a complete list of total hip arthroscopy (THA) patients that had had the prosthetic with affected catalog and lot numbers implanted. The complete list of recipients were sent a notification of the Stryker femoral head recall.
While the stated reason for the Stryker femoral head recall was taper lock failure, the prosthetic is one of several metal-on-metal models which have had allegedly caused other problems in THA patients due to the shedding of cobalt and chromium debris into the tissue surrounding the joint. This debris purportedly can cause severe pain and swelling and produce pseudotumors and even tissue death in some cases.
If the corrosion is allowed to continue, the metal debris is believed to leak metals into the bloodstream which are harmful in elevated amounts. The metals in the bloodstream—known as metallosis–ostensibly contribute to heart disease and cancer.
Symptoms of Taper Lock Failure and Metallosis
Both taper lock failure and metallosis have similar symptoms. It is difficult to tell the difference, but first and foremost, the recipient will have significant pain and swelling in the hip which affects range of motion slightly or is completely disabling. Joint instability, dislocation, and fracture usually come as a result of prosthetic loosening via the stem.
Ultimately, recipients are usually forced to undergo early revision surgery where the LFIT V40 is removed and replaced with a different prosthetic unit. This surgery alone comes with risks of blood clots, deep joint infections, bone tissue loss, and even lifelong leg length discrepancies.
If you or a loved one have been forced to have an early hip revision surgery because of the LFIT V40 femoral head or another prosthetic, you may benefit from a consultation with a knowledgeable medical device attorney.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The hip implant attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or class action lawsuit is best for you. [In general, metal hip implant lawsuits are filed individually by each plaintiff and are not class actions.] Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Stryker Metal Hip Replacement Class Action Lawsuit Investigation
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
Email any problems with this form to questions@topclassactions.com.
Oops! We could not locate your form.












