Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
Tasigna chemotherapy is popularly used throughout the United States for chronic myeloid leukemia, but recent reports indicate this drug could induce potentially fatal cardiovascular events. While Tasigna chemotherapy is still widely used in the patient population, the medical community is wary of potential cardiac complications like atherosclerosis and other circulation issues.
Tasigna (Ninotinb) was approved by the FDA in 2007 with a black box warning regarding other side effects. Tasigna chemotherapy earned manufacturer Novartis $1.7 billion in 2016. Tasigna chemotherapy works by blocking tyrosine kinase protein Bcr-Abl, which is a protein that can stimulate the growth of cancer cells.
These proteins are made by chronic myeloid leukemia cells that have an unusual feature called the Philadelphia chromosome, making Taisgna chemotherapy the ideal choice for this particular cancer. It is important to note that 95% of patients with myeloid leukemia have the Philadelphia chromosome, so it is likely most of these patients will opt for Tasigna chemotherapy.
Overview of Tasigna Chemotherapy Risks
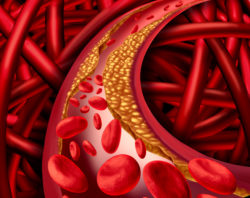
Numerous studies have indicated this cancer treatment drug may have serious side effects including the clogging and swelling of arteries. Also known as atherosclerosis, this condition occurs when the artery walls thicken and harden while plaque buildups up around them. If allowed to progress, this condition could evolve into peripheral arterial disease, (PAD), a condition in which the arteries become too small and narrow for the circulation system to properly operate. It is important to note that peripheral arterial disease is a permanent condition, forcing patients to be vigilant for any signs or symptoms for the rest of their lives.
In addition to PAD, atherosclerosis can also cause blood clot formation due to plaque that becomes weakened or inflamed. This may cause the plaque to break apart and form a blood clot. If left untreated, blood clots that remain in the peripheral arteries in the legs can become painful, create ulcers, and cause movement difficulty. If total circulation to the leg or foot is lost, the need for amputation may result. In the worst cases the blood clot forms in a cartoid artery, ultimately leading to a stroke. Even though these are very serious complications, it was not until recently that the FDA issued a warning update for the Tasigna warning label to include atherosclerosis.
The FDA issued this order in 2013, when nine Tasigna atherosclerosis studies were published, including a post market review from the FDA. This review analyzed different Tasigna injury reports from the United States, Canada, and Europe.
The FDA stated the findings of these studies had strongly implied an “association between nilotinib (Tasigna) and PAOD (peripheral arterial occlusive disease)”. Class action lawyers are currently reviewing injury claims from patients who suffered atherosclerosis as the alleged result of undergoing Tasigna chemotherapy.
Patients complain that they were not warned the cancer treatment drug could cause blood flow restrictions in vital areas of their body or pose the risk of lower limb amputation and death. Manufacturer Norvartis allegedly knew their cancer treatment drug had increased the risk of cardiovascular problems, but failed to warn patients.
In general, Tasigna lawsuits are filed individually by each plaintiff and are not class actions.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual Tasigna lawsuit or Tasigna class action lawsuit is best for you. Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Tasigna Lawsuit Investigation
If you suffered from a serious side effect or a loved one died while taking Tasigna, you may have a legal claim. See if you qualify to pursue compensation and join a free Tasigna lawsuit investigation by submitting your information for a free case evaluation.
An attorney will contact you if you qualify to discuss the details of your potential case.
Please Note: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client, if you qualify, or getting you dropped as a client.
Oops! We could not locate your form.