Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
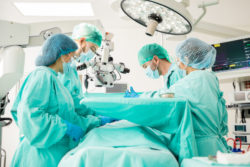
These patients who developed infection following heart surgery often needed intense medical treatment, due to the deadly nontuberculous mycobacterium (M. chimaera) festering in the surgical site.
The Sorin 3T cardiac heater cooler device is used to control a patient’s body temperature during surgery. It consists of water tanks filled with temperature controlled water. While the water does not contact the patient directly, the FDA states there is potential for contaminated water to aerolize, putting contaminants in the air of the operating room. This significantly increases the risk of infection following heart surgery, which can potentially result in death.
Overview of Cardiac Heater Cooler Infection
Recent studies have suggested higher risks of infection following heart surgery, due to scientists finding DNA evidence indicating the bacterium to be M. chimaera.
According to a new study in The Lancet Infectious Disease, it is important for patients to understand how this alleged outbreak was discovered on the device and how it may increase the risk of infection following heart surgery.
The M. chimaera problem was discovered in patients in the United States and Switzerland who contracted these bacterial infections. Investigators discovered the bacterial infection was linked to the water in tanks used to run the device during heart surgery.
The CDC warned that the M. chimaera bacterial infections were linked to the Sorin 3T cardiac heater cooler in October 2016. The CDC discovered these devices could have been contaminated at some point during the manufacturing process. If patients were to develop infection following heart surgery, it could potentially result in fatal conditions.
Researchers had examined 24 samples extracted from 21 affected patients in Switzerland, Germany, the Netherlands, and the United Kingdom. The team compared the patient samples with samples from the LivaNova cardiac heater cooler devices, along with another German company that manufactures a similar device. The researchers found the samples from the machines had much in common with those from the patients.
LivaNova responded negatively to the study. A spokesperson for the company said the study expressed more certainty than was warranted by the data.
Ever since the M. chimaera bacterial contamination was discovered, LivaNova has been facing criticism for not warning patients at an earlier time.
The FDA issued a public safety warning regarding cardiac heater cooler infections in October 2015, stating that it had received 32 Medical Device Reports (MDR) indicating patients developed infection following heart surgery. The FDA issued a later update in June 2016, with the agency specifically identifying the 3T cardiac heater cooler devices.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The cardiac heater-cooler attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or class action lawsuit is best for you. [In general, cardiac heater-cooler lawsuits are filed individually by each plaintiff and are not class actions.] Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Cardiac Heater-Cooler System Class Action Lawsuit Investigation
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
Please Note: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client, if you qualify, or getting you dropped as a client.
Email any problems with this form to questions@topclassactions.com.
Oops! We could not locate your form.












