Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
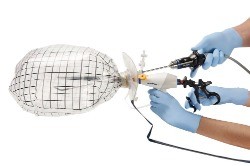
Power morcellators can be used during laparoscopic surgery to cut up larger pieces of tissue into smaller pieces that are easier to remove.
The problem at the heart of the controversy is that these devices may leave behind fragments of cancerous tissue that can spread and thrive elsewhere in the body.
This risk can be particularly high for women undergoing surgery for hysterectomy or removal of uterine fibroids. According to the FDA, about 1 in 350 of these women has a uterine sarcoma that goes undetected prior to surgery.
In April 2016, the U.S. Food and Drug Administration announced its approval of PneumoLiner, a morcellator container system designed to isolate uterine tissue that is not suspected to contain cancer. The device is manufactured by Ireland-based Advanced Surgical Concepts.
The new morcellator container system consists of a containment bag and a plunger used to position the bag during surgery.
The bag is sealed and inflated around the tissue to be removed, which is then morcellated inside the bag.
According to the FDA’s announcement, laboratory testing showed the bag is impermeable to material similar in molecular size to cells, tissues and bodily fluids.
The bag also stood up to forces that exceeded those that could be expected to occur during normal clinical use, the agency said.
Morcellator Container System Remains Unproven, FDA Says
Although the FDA considers PneumoLiner effective for containing tissue, the agency acknowledges it has not been proven to reduce the risk of spreading potentially cancerous tissue during power morcellation.
According to Dr. William Maisel, deputy director for science and chief scientist at the FDA’s Center for Devices and Radiological Health, “[t]he PneumoLiner is intended to contain morcellated tissue in the very limited patient population for whom power morcellation may be an appropriate therapeutic option – and only if patients have been appropriately informed of the risks.”
Dr. Maisel says the FDA maintains its prior position on the risks associated with power morcellation – namely, that use of a power morcellator is not advised for the vast majority of women undergoing hysterectomy or fibroid surgery.
The FDA says it will require the PneumoLiner to bear a boxed warning that is similar to the one required for laparoscopic power morcellators, alerting patients and care providers to the risk of spreading potentially cancerous tissue.
Labeling for the morcellator container system must also contain advisories for care providers reminding them of patient populations for whom the FDA says use of the device is contraindicated – specifically, women who are peri- or post-menopausal and those who are candidates for en-bloc tissue removal.
Previous studies of the use of containment bags show they are not a perfect solution to the risk of morcellation cancer.
A study published in February 2016 in the American Journal of Obstetrics and Gynecology found fluid leakage in seven out of 76 cases studied in which a containment bag was used. The report did not specify which containment bags were used in the study.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The morcellation cancer attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or class action lawsuit is best for you. [In general, morcellator cancer lawsuits are filed individually by each plaintiff and are not class actions.] Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Morcellation Cancer Class Action Lawsuit Investigation
If you or a loved one were diagnosed with cancer in the uterus, pelvis or abdomen within two years of undergoing surgery for a myomectomy (removal of fibroids), hysterectomy (removal of the uterus), oophorectomy (removal of the ovaries), or salpingectomy (removal of fallopian tubes), you may have a legal claim. See if you qualify by filling out the short form below.
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
Please Note: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client, if you qualify, or getting you dropped as a client.
Oops! We could not locate your form.












