Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
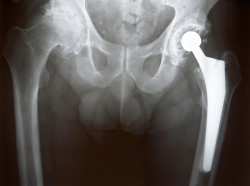
Plaintiff Doyle T. originally underwent hip replacement surgery in his right hip in August 2009, he says. He says he later began experiencing limitation of mobility and pain near the site of his implant.
His physician suspected these symptoms were caused by elevated cobalt and chromium levels in his body caused by the metal on metal design of the M2A Magnum.
Doyle says his complications worsened to the point of failure, he says. He says he finally had to undergo revision surgery to resolve his symptoms.
Doyle says that if it weren’t for representations by Biomet that the M2A Magnum was a safe and effective implant, neither he nor his orthopedic surgeon would have chosen to use that implant in Doyle’s surgery.
He attributes his hip replacement surgery failure and the resulting pain and economic loss to “the defective design, manufacture and composition of the M2A Magnum Hip System” and the lack of warning about its potential complications.
According to Doyle’s Biomet lawsuit, the Biomet M2A Magnum hip replacement system is unique among hip replacement implants in that it does not use a liner between the femoral head and the acetabular cup.
Instead, the M2A Magnum uses a “monoblock” system that “forces metal to rub against metal with the full weight and pressure of the human body.”
This design causes the implant to shed particles of cobalt and chromium into the surrounding tissue, Doyle says. The loose metal stimulates the body to attempt to reject the implant, often resulting in pain and looseness or dislocation of the joint.
It can also cause accumulation of fluid and the death of the bone and other tissue surrounding the joint, Doyle says.
Plaintiff: M2A Magnum Has a Record of Hip Replacement Surgery Failure
Doyle accuses Biomet of over-promoting the M2A Magnum system. He says that on many occasions, Biomet representatives met with orthopedic surgeons throughout the U.S.
In these meetings, Doyle alleges, the representatives told the surgeons that the M2A Magnum was a safe product with an excellent track record and a low rate of hip replacement surgery failure.
Doyle says that in these meetings, Biomet representatives neglected to reveal what the company knew about reports of complications associated with the M2A Magnum.
He says that since the implant entered the market, Biomet has received hundreds of reports of hip replacement surgery failure and other complications. He notes that the FDA has received more than 350 reports of adverse events associated with that particular implant.
Doyle argues that at the very least, Biomet should have stopped marketing the M2A Magnum once the company became aware that the implant had failed catastrophically in several patients.
His Biomet lawsuit raises strict liability claims alleging defective manufacture, defective design, failure to conform to representations and failure to warn.
He also raises claims for negligence, breach of express and implied warranties, negligent misrepresentation, fraudulent misrepresentation, fraudulent concealment and violation of Arkansas consumer protection laws.
The Biomet Hip Replacement Surgery Failure Lawsuit is Case No. 3:12-md-02391-RLM-CAN in the U.S. District Court for the Northern District of Indiana, South Bend Division.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The hip implant attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or class action lawsuit is best for you. [In general, metal hip implant lawsuits are filed individually by each plaintiff and are not class actions.] Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Metal Hip Replacement Class Action Lawsuit Investigation
If you or a loved one had a metal-on-metal hip implant that failed or caused serious complications, you may be entitled to compensation. Hip replacement lawsuits are being filed now against multiple companies, including Stryker, Biomet, DePuy, Zimmer, and Wright. See if you qualify to take legal action by filling out the form below.
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
Please Note: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client, if you qualify, or getting you dropped as a client.
Oops! We could not locate your form.












