Blood pressure med recalls overview:
- Who: Glenmark Pharmaceuticals and American Health Packaging recalled 141 batches and 21 batches of Potassium Chloride Extended-Release Capsules, USP (750 mg) 10 mEq K, respectively.
- Why: The recall was initiated over concerns the medication has a failed dissolution, which could cause patients to develop high potassium levels.
- Where: The recalled medication was distributed nationwide to wholesale, distributor and retail outlets.
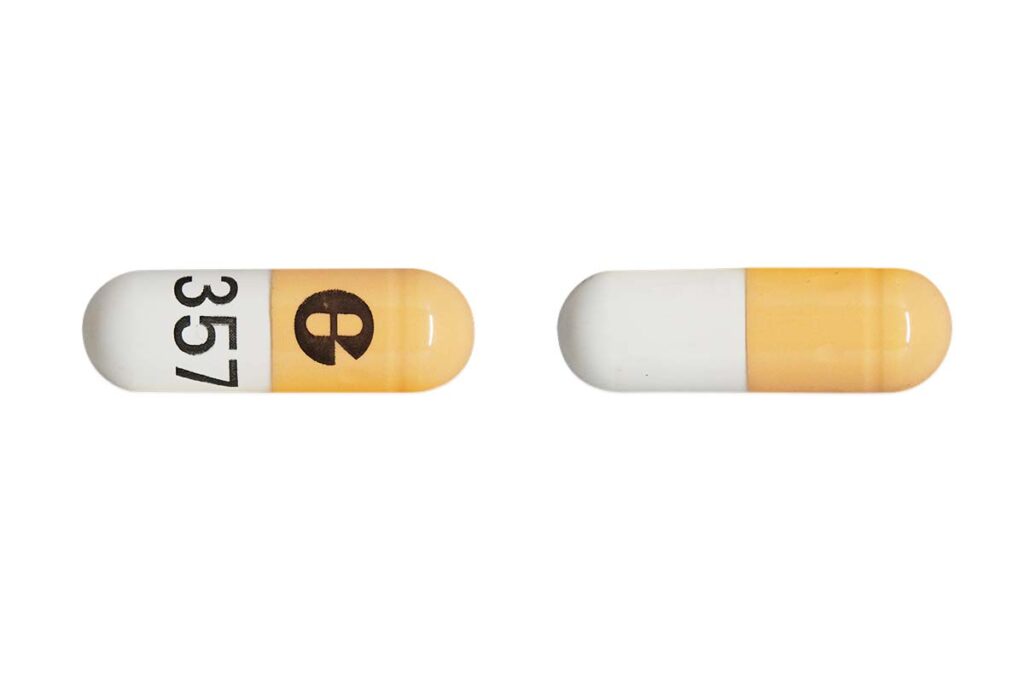
A pair of recalls were issued last month for potassium chloride extended-release capsules used to help control blood pressure, over concerns of failed dissolution.
Glenmark Pharmaceutical recalled 114 batches and American Health Packaging recalled 21 batches of Potassium Chloride Extended-Release Capsules, USP (750 mg) 10 mEq K to the consumer level.
“The failed dissolution of potassium chloride extended release capsules may cause high potassium levels, also known as hyperkalemia, which can result in irregular heart beat that can lead to cardiac arrest,” the recalls state.
The recalled medication is used to treat patients who suffer from low potassium and has been distributed nationwide to wholesale, distributor and retail outlets, according to the recalls.
Consumers should consult with doctor before stopping use of recalled medication
Consumers who take the recalled medication are advised to consult with their doctor or health care provider prior to stopping use of the product and let them know if they experience any problems that could be related to taking the medication, according to the recalls
Wholesalers, distributors and retailers, meanwhile, are advised to discontinue distributing the recalled medication immediately and to conduct a sub-recall for retail or pharmacy customers.
Both Glenmark Pharmaceuticals and American Health Packaging said they have not gotten any reports of hyperkalemia or other “serious adverse events” from spontaneous sources related to the recalls at this time.
Anyone who experiences adverse reactions or quality issues with the use of the recalled medication can report the problems to the U.S. Food and Drug Administration’s MedWatch Adverse Event Reporting program, according to the recalls.
Consumers with more questions about the recall can contact recall solutions vendor Sedgwick by phone at 1-855-695-8564 or Inmar Rx Solutions at 1-877-883-9273 to obtain further info and return instructions.
A pair of consumers filed a class action lawsuit against HoMedics and Walmart at the end of last year over claims the companies’ Equate-branded blood pressure monitors have provided inaccurate readings for thousands of users.
Are you affected by the blood pressure meds recalls? Let us know in the comments.
Don’t Miss Out!
Check out our list of Class Action Lawsuits and Class Action Settlements you may qualify to join!
Read About More Class Action Lawsuits & Class Action Settlements:















27 thoughts onBlood pressure med recalls issued over potential failed dissolution
Yes
Please add me
Add me
Add me
Please add me
Add me
Add me