Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
Tens of millions of Americans undergo surgery each year, and tens of thousands of them end up with surgical staples.
Too often, those surgical staplers malfunction in some way and result in surgical staple injuries leading to surgical staple lawsuits, or in the worst cases, even leading to death.
The U.S. Food and Drug Administration (FDA) put out an open letter last year acknowledging an uptick adverse events associated with the use of surgical staplers for internal use — not just to close external incisions – and announced it was working on several initiatives to increase the safety of their use.
Improving the safety of surgical staplers and implantable staples is a top priority for the FDA, the agency said, especially after the recall of certain staplers in 2019.
Device Classification
In its letter on March 8, 2019, the FDA said it planned to draft new labeling recommendations for stapler manufacturers and to hold a public advisory committee meeting to discuss, among other things, reclassifying the devices in order to exert more control over their testing and labeling.
In April 2019, the agency formally proposed the reclassification. The matter is still pending.
The FDA classifies medical tools and devices based on their potential danger to patients.
Currently, surgical staplers are classified as Class I devices. Other Class I devices include tongue depressors and surgical trays. These devices are deemed to be of minimal risk to patients.
As surgical staplers have the potential to cause life-threatening injuries, the FDA may change the classification to Class II or Class III. Items in these classes are subject to stricter regulation than items in Class I.
Injuries Caused by Surgical Staplers
Internal surgical staplers are a tool of efficiency. Using them to secure and connect tissue and organs can take significantly less time than traditional internal suturing, according to Healthline, and that means patients have shorter procedures and are exposed to less anesthesia.
Conversely, the staples and staplers can fail.
Among the most common and least serious of the reported malfunctions and injuries are “opening of the staple line or malformation of staples, misfiring, difficulty in firing, failure of the stapler to fire the staple, and misapplied staples (e.g., user applying staples to the wrong tissue or applying staples of the wrong size to the tissue),” the FDA’s open letter said.
Even simple malfunctions can lead to longer surgical procedures and in some cases necessitate additional surgical interventions. Those, in turn, put patients at greater risk for bleeding, infection, sepsis, damage to internal tissue and organs, the recurrence of some cancers, and death, the agency noted.
The staples are often used during minimally invasive surgeries and are intended to cut and quickly seal tissue and vessels, Kaiser Health News reported.
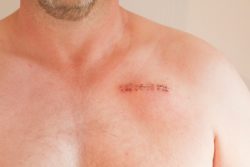
Patients have been gravely harmed when staplers have failed to fire or seal tissue, suffering from massive bleeding or infections if stomachs or intestines aren’t sealed properly.
The FDA analyzed 109,997 stapler incidents between 2011 and October 2019; those incidents included 412 deaths, 11,181 serious injuries, and 98,404 malfunctions, according to the Emergency Care Research Institute (ECRI).
Previously, the FDA acknowledged that in 2016, even though fewer than 100 stapler-related injuries were posted in the public MAUDE database, the agency had nearly 10,000 reports entered into its lesser-known internal database, Kaiser Health News reported. Between 2011 and 2018, a reporter found millions of hidden reports of medical device injuries, including more than 56,000 previously undisclosed device injuries were found in this internal database.
That database may have made it difficult for doctors and patients to make informed decisions on whether to use these medical devices. According to doctors who experienced issues with surgical staplers, looking through medical databases to find out if these malfunctions were common yielded few results, Kaiser Health News reported.
Patients have also described feeling alone due to the secrecy surrounding stapler malfunctions.
One woman who spoke to the Tampa Bay Times recalled feeling like the only person who had gone through what she had. After surgical staples that were used to seal her colon during surgery failed, she suffered from a colon leak that required additional surgeries and a prolonged recovery.
She decided to file a lawsuit against the manufacturer of her staples after it was revealed that a database full of surgical staple injury cases had been hidden from public view, according to the Tampa Bay Times. Had it been known that surgical staplers had the potential to cause life-threatening injuries and potentially wrongful death, she and other patients may have chosen another surgical method.
Ethicon Stapler Recall
Ethicon, a Johnson & Johnson subsidiary, has been subject to multiple surgical stapler recalls over concern of surgical staple injury.
Several cases of reported surgical staple injury prompted the FDA in October 2019 to announce a Class I recall of several Ethicon surgical staplers.
Four of Ethicon’s Echelon Flex Endopath staplers were recalled due to a potential flaw in the jaw of the device that could lead to malformed staples, the FDA said.
A report on the recall published by the Regulatory Affairs Professionals Society noted there had been seven serious injuries and one reported death linked to the Ethicon staplers.
The recall affected staplers in other countries in addition to the United States, according to Mass Device.
Ethicon issued a public notice stating the defective staplers were distributed in Germany, Luxembourg, Switzerland, Denmark, the Netherlands, and Belgium. The staplers included in the recall were manufactured beginning Aug. 1, 2019, according to Mass Device.
Ethicon has since halted the production of defective lots, and has since taken steps to identify the source of the defect and figure out how to prevent it from occurring again.
The October 2019 recall came on the heels of another Class I recall of Ethicon staplers in May 2019.
That recall involved a different kind of device.
Where the Echelon Flex Endopath is a linear stapler, the previously recalled staplers are circular staplers: the Endo-Surgery Curved Intraluminal Stapler with adjustable-height staples and the Endo-Surgery Endoscopic Curved Intraluminal Stapler with adjustable-height staples.
An investigation into the alleged issues with the circular staplers indicated that the problems could have been associated with a change in the manufacturing process for the devices, according to the FDA. This change to the process reportedly led them to produce malformed staples, which could cause injury to patients.
The change was implemented in March 2018, and the new process was in use through March of the following year.
A growing number of patients are pursuing litigation against Ethicon and Johnson & Johnson, alleging that the company’s surgical staples were defective and caused serious complications.
If you or someone you love has undergone surgery utilizing an Ethicon surgical stapler only to develop complications, you may be able to file a lawsuit and pursue compensation. Many patients may not know if an Ethicon surgical stapler was used during their surgery, but there are ways to find out.
Filing a lawsuit can be a daunting prospect, so Top Class Actions has laid the groundwork for you by connecting you with an experienced attorney. Consulting an attorney can help you determine if you have a claim, navigate the complexities of litigation, and maximize your potential compensation.
Join a Free Ethicon Surgical Stapler Lawsuit Investigation
If you or a loved one suffered from surgery complications caused by internal staples, you may qualify to joing in a Ethicon surgical stapler lawsuit investigation.
See if you qualify by filling out the free form on this page.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Ethicon Surgical Stapler Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
Questions@TopClassActions.com.
Oops! We could not locate your form.