Patients who are taking Xeljanz for the rheumatoid arthritis should be aware of some of the medication’s side effects, which range from minor issues to life-threatening cardiovascular events.
In addition, Xeljanz has been associated with an elevated risk of illnesses that include some forms of cancer. The U.S. Food and Drug Administration (FDA) recently issued a safety alert, warning the public about the dangers of higher dosages of Xeljanz.
What is Xeljanz?
Xeljanz (tofacitinib) is part of a class of prescription drugs known as Janus kinase inhibitors, or JAK inhibitors. These drugs prevent the action of certain enzymes (known as JAKs) that are believed to play a role in autoimmune diseases such as rheumatoid arthritis.
Why is Xeljanz Prescribed?
Xeljanz was originally approved by the FDA in November 2012 for the treatment of “moderately to severely active rheumatoid arthritis” in patients who fail to respond to other treatments. Due to the drug’s potential side effects, it is not meant to be prescribed as a first line of treatment for sufferers of rheumatoid arthritis (RA).
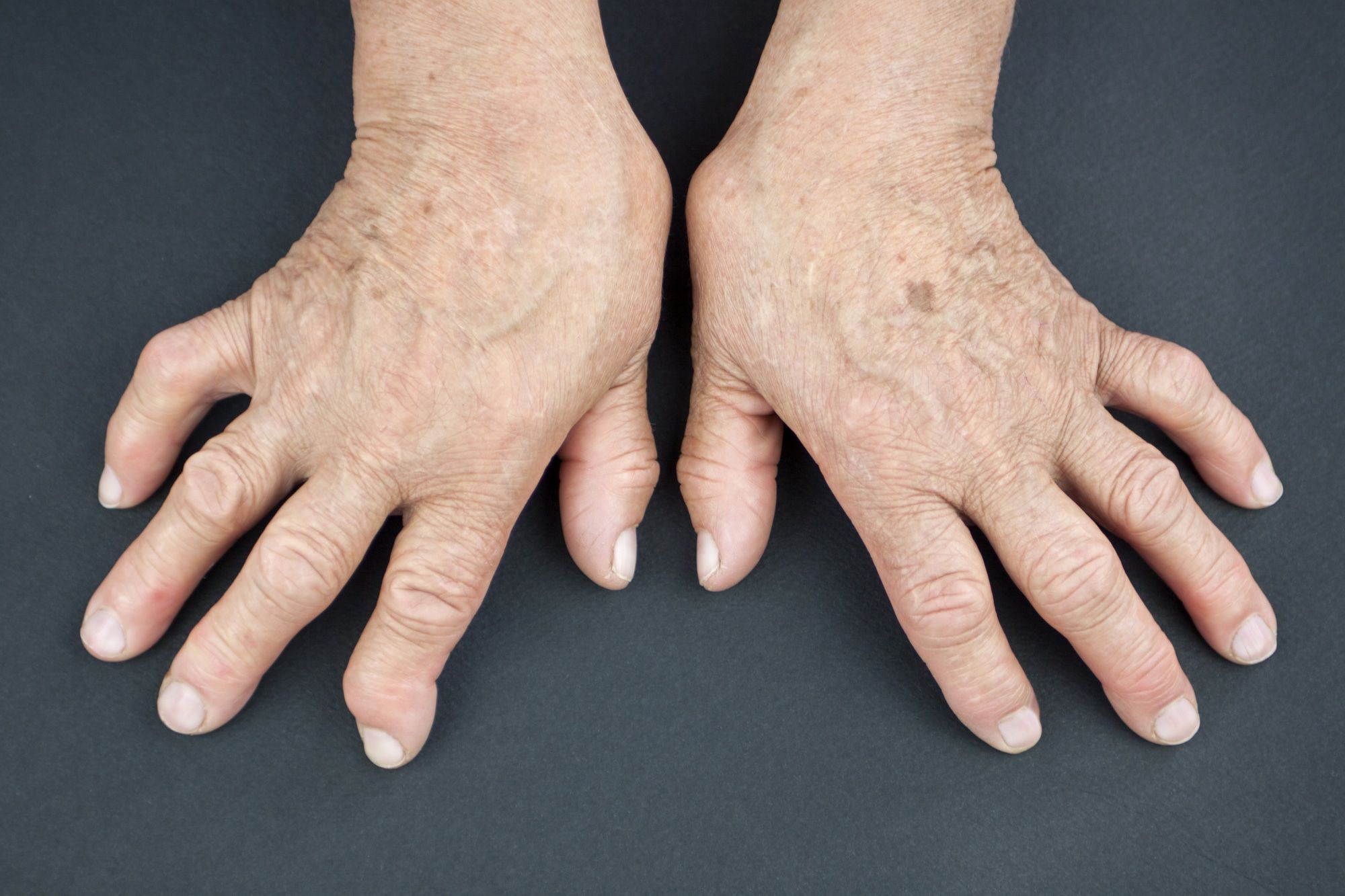
Rheumatoid arthritis is a condition that primarily affects the joints, as well as the skin, lungs, eyes, heart, and blood vessels. The disease is chronic, and as an autoimmune disorder, it occurs when the immune system begins to attack the body’s own tissues.
Rheumatoid arthritis causes the lining of the joints to swell, which can result in tender or warm joints, joint stiffness, fatigue, fever, and loss of appetite. If the swelling goes untreated, the condition may result in bone erosion, joint deformity, and joints shifting out of place. The inflammation caused by rheumatoid arthritis can even destroy the cartilage and bone within the joints.
The condition usually begins in smaller joints, such as the fingers or toes. As it progresses, it may begin to affect the wrists, knees, ankles, elbows, hips, and shoulders. In many cases, rheumatoid arthritis may come and go in what are called flares.
Xeljanz treats other autoimmune diseases in addition to RA. In May 2018, the drug won approval as a treatment for ulcerative colitis. The drug may also be prescribed for psoriatic arthritis.
Additional uses may be forthcoming. Currently, Xeljanz is being investigated for the treatment of:
- Alopecia areata
- Ankylosing spondylitis
- Atopic dermatitis
- Psoriasis
- Vitiligo
Data published in the New England Journal of Medicine shows that Xeljanz may even be useful in treating COVID-19. The drug reduced the risk of respiratory failure or death in hospitalized COVID-19 patients, according to the data.
However, despite its wide range of potential uses, Xeljanz comes with significant side effects.
What are Common Side Effects of Xeljanz?
Minor side effects commonly reported with Xeljanz are headaches, diarrhea, infections of the upper respiratory tract, and cold-like symptoms (nasopharyngitis).
What Are the More Serious Side Effects?
Patients on Xeljanz may exhibit symptoms of jaundice, pale skin, respiratory distress and histamine reactions or anaphylaxis, loss of color, and pale-colored stools. Another serious concern is the increased chance of blood clotting, or embolism, which can lead to stroke and heart attacks. These risks appear to be dose-dependent; patients taking Xeljanz at the 10 mg dosage were significantly more likely to experience side effects than those taking 5 mg, according to study results.
 There have also been safety concerns about elevated cancer risk (primarily lymphoma), abnormally high white blood count, hypertension, liver damage, and bleeding stomach ulcer. It was because of these risks that the drug was rejected by the European Medicines Agency, which determined that its efficacy was no greater than that of alternative treatments.
There have also been safety concerns about elevated cancer risk (primarily lymphoma), abnormally high white blood count, hypertension, liver damage, and bleeding stomach ulcer. It was because of these risks that the drug was rejected by the European Medicines Agency, which determined that its efficacy was no greater than that of alternative treatments.
Additionally, nearly half of patients who begin taking Xeljanz for the control of their arthritis stop taking the drug after a year. This high discontinuation rate for a drug meant to help manage a long term condition may be due to the potential for dangerous side effects, the level of effectiveness of the drug, or the price tag.
Recent clinical trial results support concerns surrounding Xeljanz. In late January 2021, Pfizer released co-primary results from its post-marketing safety study. According to these results, Xeljanz patients may be at an increased risk of cardiovascular events and cancer. Shortly after, Health Canada followed in the footsteps of European regulators when it announced its own safety review of Xeljanz.
Has the FDA Said Anything About Xeljanz Risks?
The FDA released a safety alert in February 2019, notifying the public that it would be investigating Xeljanz for the risk of blood clots in the lungs. This clotting condition, known as pulmonary embolism, can have serious complications and can be life-threatening if left untreated.
An ongoing safety trial reportedly revealed that patients were at an increased risk of pulmonary embolism when treated with a 10 mg twice daily dose of Xeljanz. Although Xeljanz is approved to treat both rheumatoid arthritis and ulcerative colitis, this twice-daily dose is approved only to treat ulcerative colitis.
Due to the risks associated with the twice-daily dosage of Xeljanz, the FDA said it would be investigating the blood clotting risk in the future.
In July, the FDA updated the situation by approving new warnings for Xeljanz regarding the risk for blood clots and death.
“We approved these changes, including adding our most prominent Boxed Warning, after reviewing interim data from an ongoing safety clinical trial of tofacitinib in patients with rheumatoid arthritis (RA) that examined a lower and this higher dose of the medicine,” the FDA stated in its safety announcement.
The agency also applied stricter requirements for prescribing Xeljanz for treating ulcerative colitis. Now, the drug will only be prescribed to treat ulcerative colitis if patients are not treated effectively by other drugs or to those who experience extreme side effects with other medications.
Should I Stop Taking Xeljanz?
It is not advised to stop taking any prescription medication without consulting your physician. Doing so may result in a worsening of your condition. If consumers are concerned about the risks, they can speak to their doctor about changing medications in a supervised manner.
However, if you are experiencing any symptoms of blood clotting, it’s a good idea to seek the help of a health care professional as soon as possible.
What Else Should I Know About Xeljanz?
Currently, Xeljanz’s manufacturer, Pfizer, is the subject of investigations into whether or not it was aware of these dangerous side effects and failed to inform patients and the medical community. The FDA has ordered Pfizer to continue its clinical trials and safety evaluations of the medication at different dosages through the end of the current year.
Join a Free Xeljanz Blood Clot Lawsuit Investigation
If you or someone close to you suffered a pulmonary embolism, deep vein thrombosis, or died after taking Xeljanz or Xeljanz XR, you may benefit from participating in a free Xeljanz blood clot lawsuit investigation. Learn more by filling out the short form on this page.
This article is not legal advice. It is presented
for informational purposes only.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2025 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Xeljanz Blood Clot Lawsuit Investigation
If you qualify, an attorney will contact you to discuss the details of your potential case at no charge to you.
PLEASE NOTE: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
E-mail any problems with this form to:
[email protected].
Oops! We could not locate your form.












