Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
NuvaRing Lawsuits Postponed, Opening Door for New Cases
By Christina Drury
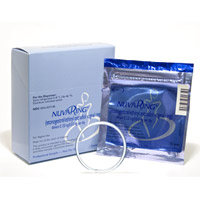 There has been yet another delay in the proceedings involving NuvaRing lawsuits. This latest development has all involved wondering if the manufacturer – originally Organon, now Merck – is ready to start settlement talks. If so, this could bring relief to over 1,000 plaintiffs who have filed NuvaRing lawsuits.
There has been yet another delay in the proceedings involving NuvaRing lawsuits. This latest development has all involved wondering if the manufacturer – originally Organon, now Merck – is ready to start settlement talks. If so, this could bring relief to over 1,000 plaintiffs who have filed NuvaRing lawsuits.
The trouble for NuvaRing began in 2007, when the filing of lawsuits over the marketing of NuvaRing and its sale became a cause for concern. Since then there have been over 4,500 adverse NuvaRing side effects reported and numerous medical studies published that link the use of NuvaRing and similar birth control products containing the same hormones to an increased risk of potentially dangerous side effects.
History of NuvaRing
NuvaRing is a vaginal form of birth control that is inserted into the vaginal area once a month. It releases a low dosage of estrogen and etonogestrel over the course of a three-week period. When it was first approved by the U.S. Food and Drug Administration (FDA) in 2001, it was marketed as a device that would make birth control healthier and simpler than taking birth control pills, which have to be taken every day at around the same time in order to reach maximum effectiveness.
From there, the problems for NuvaRing and new manufacturer, Merck, began to snowball. A study published in 2009 by The British Medical Journal linked the use of NuvaRing and other birth control devices using third- and fourth-generation progestin – a synthetic hormone – to an alarming increase in developing dangerous blood clots. The progestin in NuvaRing is relatively new and was formulated as a means of helping to control the development of acne and growth of hair that is commonly associated with the use of progestin.
Despite Medical Studies Confirming NuvaRing Blood Clot Risks, Sales Skyrocket
Drawn in by the advertising that claims NuvaRing will add to the simplicity of life since it is used only once monthly, over 5.5 million NuvaRing prescriptions were written for women in 2010. The sale of the device led to $559 million in sales internationally in 2010 alone.
Fast forward to October of 2011, when the FDA funded a medical study that revealed 56% of women using NuvaRing were subjected to an increased risk of suffering pulmonary embolism and deep vein thrombosis, compared to earlier forms of birth control.
The problems only continued for NuvaRing in 2012 when an additional study, also published in The British Medical Journal, revealed that NuvaRing users were more than 6.5 times likely to develop NuvaRing side effects such as blood clots as opposed to women who took no birth control at all. The most shocking determination of this study revealed that NuvaRing users were 90% more likely to develop dangerous blood clots than women who were taking other forms of oral contraceptives that were designed with older forms of hormones.
NuvaRing Lawsuits Ongoing
The delay in litigation has opened the door for more women to participate in NuvaRing lawsuits. For more information on the NuvaRing side effects, NuvaRing lawsuits and how you can submit your personal information to qualified attorneys, visit our NuvaRing Birth Control Class Action Lawsuit Settlement & Investigation page.
Updated January 22nd, 2013
All medical device, dangerous drug and medical class action and lawsuit news updates are listed in the Drug and Medical Device section of Top Class Actions.
Top Class Actions Legal Statement