Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
Vaginal Mesh Implant Lawsuit Update: Ethicon Files Answer in Federal Court
By Andrea Gressman
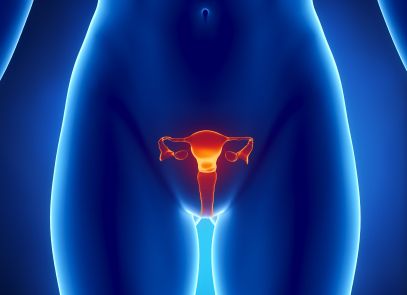
On August 31, 2012, an “Answer to the First Master Amended Complaint” was filed by Ethicon, Inc. in this federal vaginal mesh implant lawsuit, which is now ongoing in the Southern District of West Virginia’s U.S. District Court. Ethicon is a division of Johnson & Johnson and, according to one law group, is facing hundreds of vaginal mesh implant lawsuits regarding Ethicon transvaginal mesh implant products. In the Answer filed with the Court in August, several of Ethicon products are named in the vaginal mesh implant lawsuit, including: Prolene Mesh/ Prolene Soft Mesh, Gynemesh, Gynemesh PS, TVT, TVT- Obturator, TVT- SECUR, TVT- Exact, TVT Abbrevo, Prolift, Prolift M+ and Prosima products.
The case is known as In re: Ethicon Inc., Pelvic repair System Products Liability Litigation, MDL No. 2327.
In the past few years, more and more women have come forward with complaints of often painful complications after placement of transvaginal mesh implants for the treatment of stress urinary incontinence and pelvic organ prolapse, bringing Ethicon under scrutiny.
Between 2008 and 2012, the U.S. Food & Drug Administration (FDA) received in excess of 1,500 reports of transvaginal mesh implant side effects between 2008 and 2010. These side effects include erosion of the mesh into the vaginal wall, contraction of the mesh, infection at the site, urinary complications, pain in the pelvis, scarring of the vaginal wall and more. The FDA noted at the time of this report that the problems were classified as “not rare.”
Additional Vaginal Mesh Implant Lawsuits
On top of the litigation against Ethicon, there are a significant number of other large actions out against other transvaginal mesh implant and bladder sling implant manufacturers. These are all currently pending in the Southern District of West Virginia. These other vaginal mesh implant lawsuits include American Medical Systems Inc., Pelvic Repair Systems Products Liability Litigation (MDL No. 2325); Boston Scientific Corp., Pelvic Repair Systems Products Liability Litigation (MDL No. 2326); and C.R. Bard, Inc., Pelvic Repair System Products Liability Litigation (MDL No. 2187).
Filing a Vaginal Mesh Implant Lawsuit
Patients who experienced complications or difficulty following surgical implanting of transvaginal mesh or a bladder sling implant may be eligible for compensation for their injuries, medical bills and pain and suffering. Free vaginal mesh implant lawsuit evaluations are available to any women who used an Ethicon mesh device or any other transvaginal mesh implant product and experienced complications.
Visit the Transvaginal Mesh, Vaginal Sling, Vaginal Mesh and Bladder Sling Class Action Lawsuit Investigation page today for your free consultation. Vaginal mesh implant lawyers are currently investigating vaginal mesh implant side effects including pain, bleeding, infections, mesh erosion, perforation, urinary problems, and other vaginal mesh side effects.
Updated October 16th, 2012
All medical device, dangerous drug and medical class action and lawsuit news updates are listed in the Drug and Medical Device section of Top Class Actions.
Top Class Actions Legal Statement