Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
Testing for asbestos in baby powder can help protect consumers from contaminated talc and the related risks for cancer and other health problems. Baby powder cancer is the subject of thousands of lawsuits across the country.
Testing for Asbestos in Baby Powder
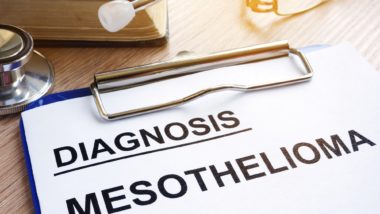
Asbestos exposure predisposes consumers to a variety of health problems, including lung cancer and mesothelioma – cancer in the layer of tissue covering most internal organs.
Unfortunately, talc and asbestos are found in the same area of the earth. The U.S. Food and Drug Administration (FDA) explains that when talc is being mined, it may become contaminated by asbestos. If the talc is not properly tested and purified, asbestos contamination may carry over to finished talc-based products such as baby powder and cosmetics.
In light of the potential risks of asbestos exposure, the FDA has dedicated significant time and effort to testing talc and talc products along with researching better ways to test these products for asbestos.
FDA Recalls
In October 2019, the FDA took action against asbestos in baby powder when it coordinated a voluntary recall with Johnson and Johnson. The manufacturer recalled a single lot of its baby powder after testing revealed the presence of asbestos fibers.
“I understand today’s recall may be concerning to all those individuals who may have used the affected lot of baby powder,” acting FDA Commissioner Ned Sharpless said in the recall announcement. “I want to assure everyone that the agency takes these concerns seriously and that we are committed to our mandate of protecting the public health.”
“The FDA continues to test cosmetic products that contain talc for the presence of asbestos to protect Americans from potential health risks.”
The asbestos results were reportedly obtained as part of an “ongoing survey of cosmetic products” conducted by the FDA since 2018. Two samples of Johnson and Johnson’s baby powder were tested. One of the samples yielded a positive result, while the other showed no asbestos.
 FDA Regulations
FDA Regulations
Although the FDA does not directly regulate asbestos in the United States, the agency is in charge of ensuring drugs, cosmetics, and other covered products are free of hazards, including asbestos.
Following the Johnson and Johnson baby powder recall and growing concern about talc powder safety, the FDA held a public meeting about its testing methods for asbestos in baby powder. During the meeting, a variety of experts, lawyers, consultants, organizations, and others spoke at the FDA-hosted event.
These presentations discussed the best way to test for asbestos in talc as well as the importance of a thorough examination. In its preliminary executive summary following the meetings, the FDA’s Interagency Working Group on Asbestos in Consumer Products identified ways to direct efforts to “promote reliability of the analytical methods for asbestos and other elongated mineral particles (EMPs) of health concern in talc and talc-containing consumer products.”
Conclusions from the meeting may help FDA regulations better protect consumers from the dangers of asbestos in talc products.
In March, the FDA released its final report on a year-long sampling of talc products by AMA Analytical Services Inc. – the final of six parts. The sampling assignment resulted in 43 negative samples and nine positive ones. The year-long sampling was reportedly responsible for detecting the positive Johnson & Johnson baby powder-asbestos result.
“We have taken and will continue to take swift action when we determine a cosmetic product is not safe,” Susan Mayne, director of the Center for Food Safety and Applied Nutrition, said in a statement.
For 2020, the FDA has contracted AMA to test an additional 50 samples in blind testing. Should any asbestos be detected in talc products, the FDA will communicate the results to the public. Final results from the 2020 blind testing are expected for release in early 2021.
The agency notes that this new round of testing reflects the ongoing work to better detect asbestos in talc products.
“There is general agreement among U.S. federal agencies and the World Health Organization that there is no known safe level of asbestos exposure,” Mayne said. “Our work in this area is ongoing and we’ll continue to communicate with the public as we have updates to share.”
Some victims have claimed that Johnson & Johnson knew for decades that its baby powder was contaminated with asbestos but continued to market it as a safe hygiene product for women and children despite the risks. Several plaintiffs who have filed lawsuits against the pharmaceutical company claiming that their cancers were caused by asbestos in baby powder have won their cases and received large settlement sums.
Various Organization Recommendations
Unfortunately, there is not a lot that consumers can do other than trust that manufacturers have sufficiently purified and tested their talc before manufacturing products. Most consumers don’t have access to the testing methods used to detect asbestos in talc, but may be able to take steps to protect themselves.
Generally, experts do not recommend using talcum powder products, such as baby powder or talc-based cosmetics. The American College of Obstetricians and Gynecologists advises women not to use talcum powder in the vaginal area for feminine hygiene, although it acknowledges that there is “no medical consensus that talcum powder causes ovarian cancer,” says Self.com
The American Academy of Pediatrics (AAP) also discourages the use of baby powder due to risks, stating that “if inhaled, talcum-containing powders can cause severe lung damage and breathing problems in babies.”
Dangers of Asbestos Exposure
In addition to the potential risks associated with women using asbestos-contaminated baby powder for hygiene purposes, or babies inhaling the powder, asbestos exposure has been linked to serious health conditions including lung cancer.
Inhaling asbestos particles may lead to health conditions including mesothelioma, lung cancer, laryngeal cancer, and asbestosis. These particles may settle in the lungs and other internal organs, where they can cause irritation, inflammation, and scar tissue.
The conditions caused by asbestos may take years or decades to develop, which often makes it difficult for victims to determine their point of exposure. Additionally, due to the length of time it takes these cancers to develop, it is almost always too late to reverse the damage caused by the condition by the time the cancer is diagnosed.
Asbestos-related conditions cause the deaths of at least 10,000 people in the U.S. each year, and around 4 percent of all lung cancer cases are attributable to asbestos exposure. Virtually all cases of mesothelioma are caused by exposure to asbestos particles.
$100 Million Baby Powder Settlement
Bloomberg reports that Johnson & Johnson has agreed to pay $100 million to resolve more than 1,000 lawsuits alleging that its baby powder products caused cancer. The company reportedly maintains that there is no asbestos in baby powder sold by Johnson & Johnson.
“In certain circumstances, we do choose to settle lawsuits, which is done without an admission of liability and in no way changes our position regarding the safety of our products,” a company spokesperson stated in an email to Bloomberg News. “Our talc is safe, does not contain asbestos and does not cause cancer,” she continued.
The company faces at least 20,000 other lawsuits over its talc-based baby powder products, according to Bloomberg.
Johnson & Johnson has reportedly removed talc-based baby powder products from its U.S. and Canadian markets. In addition, the company recalled products in 2019 after the FDA announced that it had found trace levels of the carcinogen in Johnson & Johnson products, flying in the face of CEO assertions that their talc and baby powder does not contain asbestos.
The company is still fighting lawsuits filed by people who allege that they developed mesothelioma and ovarian cancer after using J&J’s talc-based products. In 2018, a New York Times investigation revealed that Johnson & Johnson’s own tests had shown that the talc it used was contaminated with asbestos, but the information was withheld from the public for at least half a century.
Join a Free Johnson’s Baby Powder Class Action Lawsuit Investigation
If you or your loved one was diagnosed with ovarian cancer and used a baby powder product such as Johnson and Johnson Baby Powder or Shower to Shower, submit your information now in the form on this page for a free and confidential case evaluation.
You may qualify to pursue compensation for your medical bills, pain and suffering, and other damages.
See if you qualify by filling out the free form on this page.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Johnson’s Baby Powder Class Action Lawsuit Investigation
Failing to warn consumers about the danger of baby powder cancer could make companies liable for your injuries. If you used Johnson’s Baby Powder or Shower to Shower body powder and were diagnosed with ovarian cancer or mesothelioma — or your loved one was — you may have a legal claim.
Please Note: If you want to participate in this investigation, it is imperative that you reply to the law firm if they call or email you. Failing to do so may result in you not getting signed up as a client or getting you dropped as a client.
Submit your information now for a free case evaluation!
E-mail any problems with this form to:
Questions@TopClassActions.com.


 FDA Regulations
FDA Regulations