Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
Shortly after its approval, reports of serious issues with Risperdal side effects and the inappropriate marketing of Risperdal began to surface. To date, the manufacturers of Risperdal have paid billions in criminal penalties, fines, and Risperdal lawsuit settlements for a variety of reasons including injury to patients.
Serious Risperdal Side Effects
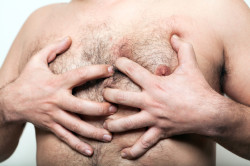
Male Breast Growth:
Gynecomastia is enlargement or development of breast tissue in males. It is more common, and particularly disturbing, in children and adolescents. The development of gynecomastia is usually a permanent change that will require surgical removal by a reconstructive surgeon. Gynecomastia has been attributed to Risperdal’s stimulation of prolactin, a hormone common in women who are pregnant or nursing. Adolescents or boys who develop breast tissue suffer not only physical changes, but they may also suffer severe emotional trauma from Risperdal gynecomastia.
Movement Disorders: Risperdal was thought to have a lower risk of movement disorders, known as extrapyramidal symptoms or side effects, than older antipsychotics. For this reason, the company allegedly downplayed the risks of Risperdal movement disorder, but adequate testing of children and widespread use for a number of psychiatric conditions has made this Risperdal side effect much more prevalent.
Diabetes: Risperdal side effects have been associated with an increased risk of development or worsening of diabetes. The exact relationship is unknown.
Death in the Elderly: Risperdal side effects have been associated with mortality in elderly dementia patients. The FDA required a Black Box warning regarding Risperdal use in the elderly and chance of death from adverse events including: cardiac failure, pneumonia and cerebrovascular events (stroke). The exact relationship is unknown and the dose of medication did not matter.
Physiological Issues: Risperdal can also cause physiological problems when taken with other medicines. These effects may include irregular heartbeat and an increased risk of seizures. Most of these Risperdal side effects and drug interactions are not severe, but any side effects can impact the well-being of a patient and may pose more risk for some.
Risperdal Lawsuits
Risperdal received approval in 1993 as the first drug of the new FDA drug category called atypical antipsychotics. By 1996, over 1 million prescriptions for the medication had been written, including many for unapproved, off-label uses. As early as 1999, reports began to surface regarding problems with Risperdal use in children, which was not an approved Risperdal use at that time.
Physicians may use medications for off-label reasons if they feel the patient will benefit; however, the manufacturer is not allowed to promote medications for unapproved indications. Janssen Pharmaceuticals and its parent company, Johnson & Johnson, have allegedly improperly marketed Risperdal for unapproved uses.
Attorneys for Risperdal victims claim that Johnson & Johnson used illegal marketing to promote Risperdal for unapproved uses even after they were aware of the Risperdal side effects of gynecomastia, movement disorders, and the risk of death, but minimized these risks and did not adequately warn consumers of those Risperdal side effects.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The Risperdal attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or Risperdal class action lawsuit is best for you. [In general, Risperdal lawsuits are filed individually by each plaintiff and are not class actions.] Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Risperdal Class Action Lawsuit Investigation
If you or your son took Risperdal between the ages of 10 and 18 years old and suffered gynecomastia (male breast growth), male breast pain, nipple pain, or nipple discharge, you may be entitled to compensation. See if you qualify by submitting your information below for a free and confidential case review.
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
Oops! We could not locate your form.












