Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
A federal judge struck down an attempt by Medtronic Inc. to upend Infuse complications lawsuits by arguing they are based on state law and should be preempted by federal law. The ruling last month allows various Infuse lawsuits to move forward.
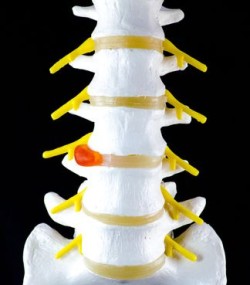
The Infuse Bone Graft System is a type of surgical implant designed to be used in spinal fusions and several other bone surgeries. Infuse consists of a metal cage and a bio-engineered gel.
This gel encourages bone to grow. In a spinal fusion surgery, the steel cage of Infuse is used to hold two adjacent vertebrae together while the gel encourages them to grow together.
Spinal fusion surgery is used to strengthen a part of the spine after a spinal disc fails. The Infuse Bone Graft System is also approved for use in certain dental and facial surgeries, but in these cases the steel cage is not used, only the bio-engineered gel.
However, Infuse lawsuits have alleged that the Infuse Bone Graft System is potentially dangerous, particularly when used “off-label,” or beyond the U.S. Food and Drug Administration’s approved uses of the device.
Allegedly, the Infuse Bone Graft System can cause swelling, making it dangerous for use in the neck. Additionally, it has been alleged that the Infuse Bone Graft System can promote uncontrolled bone growth.
In the nerve- and muscle-rich tissues around the spinal column, uncontrolled bone growth can cause serious medical problems. Uncontrolled bone growth in the spine can cause nerve damage, which in turn can cause severe pain, numbness, paralysis, and other complications. Other Infuse complications have also been alleged.
One issue that has come up in the various Infuse lawsuits is off-label usage. Under U.S. law, a physician can prescribe a medical device or drug outside of its FDA-approved purpose if, in their judgment, it could help a patient.
This leeway is intended to allow doctors to use their judgment in cases when medical research moves faster than FDA approval. But there is a caveat that medical manufacturers may not market their wares off-label.
Under U.S. law, off-label use of a medical device is permissible, but off-label marketing is strictly forbidden. Medtronic Inc. has been accused of engaging in a clandestine off-label marketing campaign for the Infuse Bone Graft System, by hiring doctors to write up “research articles” and give presentations singing Infuse’s praises, without disclosing the fact that Medtronic was paying them.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or class action lawsuit is best for you. [In general, Infuse bone graft lawsuits are filed individually by each plaintiff and are not class actions.] Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Medtronic Infuse Class Action Lawsuit Investigation
An investigation has been launched to find spinal surgery patients who were implanted with Medtronic’s Infuse bone graft and suffered complications such as nerve damage; excessive bone growth; chronic pain; difficulty breathing, swallowing, and speaking; male sterility and other uro-genital injuries. See if you qualify to take legal action by filling out the short form below.
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
Oops! We could not locate your form.