Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
This has led to over 1,000 Medtronic Infuse Bone Graft lawsuits to be filed over Infuse Bone Graft complications.
The Medtronic Infuse bone graft has been U.S. Food and Drug Administration (FDA) approved for certain spinal fusion surgeries: specifically, the anterior approach lumber fusion surgery. It has also been approved for certain dental surgeries. Medtronic has been accused of promoting the Infuse Bone Graft for cervical spinal fusion as well, which the FDA specifically stated was not approved.
It is not illegal for doctors to use the Infuse Bone Graft for off-label purposes, but it is illegal for Medtronic to promote their product for off-label uses. The medical device maker may be held legally responsible for thousands of Infuse bone graft side effects.
Infuse Bone Graft side effects include cancer, difficulty swallowing, breathing and speaking, uncontrolled bone growth, nerve injuries, chronic pain, male sterility, and cauda equina syndrome.
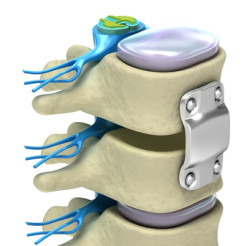
What is the Medtronic Infuse Bone Graft?
The Medtronic Infuse Bone Graft is a device that encourages bone growth in the spine. It uses a protein called rhBMP-2 to encourage growth in spinal fusion surgery. It is an alternative to the traditional bone grafting method which can also be used for spinal repair.
Infuse Bone Graft complications have been known to the medical community for quite some time. In 2013, the National Center for Biotechnology Information suggested that the Medtronic Infuse Bone Graft could cause a potentially deadly problem during revision surgery involving vascular injuries.
Medtronic allegedly bribed doctors to hide Infuse Bone Graft side effects from the public. Medtronic’s studies on Infuse Bone Graft safety supposedly hid potential safety issues. One study published in the Spine Journal suggested that 10 percent to 50 percent of patients studied experienced adverse Medtronic Infuse Bone Graft complications.
Medtronic Infuse Bone Graft Lawsuits
Hundreds of Medtronic Infuse Bone Graft lawsuits have been filed against Medtronic.
Medtronic submitted a motion to dismiss a Medtronic class action lawsuit that stated Medtronic was involved in a scheme that misled investors particularly in the area of Infuse Bone Graft side effects. It was also suggested that Medtronic’s initial studies involving Infuse Bone Graft complications were inaccurate and misleading.
The judge denied the motion to dismiss the Medtronic class action lawsuit. Medtronic’s stock suffered as a result of the allegations made in this Medtronic Infuse Bone Graft lawsuit.
Individuals who have experienced negative Medtronic Infuse Bone Graft complications such as uncontrolled bone growth, difficulty breathing, male sterility, or cancer have filed a Medtronic Infuse Bone Graft lawsuit or have joined a Medtronic class action lawsuit.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual lawsuit or class action lawsuit is best for you. [In general, Infuse bone graft lawsuits are filed individually by each plaintiff and are not class actions.] Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Medtronic Infuse Class Action Lawsuit Investigation
An investigation has been launched to find spinal surgery patients who were implanted with Medtronic’s Infuse bone graft and suffered complications such as nerve damage; excessive bone growth; chronic pain; difficulty breathing, swallowing, and speaking; male sterility and other uro-genital injuries. See if you qualify to take legal action by filling out the short form below.
An attorney will contact you if you qualify to discuss the details of your potential case at no charge to you.
Oops! We could not locate your form.












