Top Class Actions’s website and social media posts use affiliate links. If you make a purchase using such links, we may receive a commission, but it will not result in any additional charges to you. Please review our Affiliate Link Disclosure for more information.
Kombiglyze XR combines saxagliptin and metformin and is classified as an incretin mimetic, which works by mimicking metabolic hormones (incretins) and increasing GLP-1, a hormone that stimulates the pancreas to secrete more insulin.
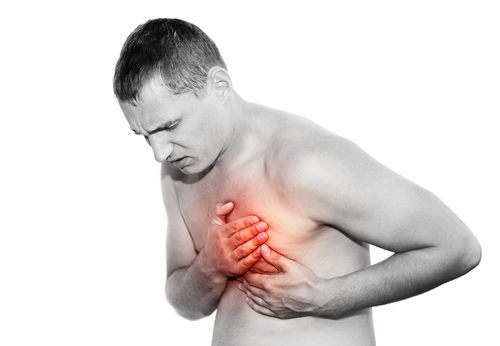
However, the new type-2 diabetes treatment has been reportedly linked to serious Kombiglyze XR side effects, including heart failure.
Kombiglyze XR Side Effects
The problem with any new medication is that sometimes there are unanticipated side effects that were not found in the early clinical trials. Kombiglyze belongs to a relatively new class of type-2 diabetes treatment medications that, according to the latest diabetes news, have been increasingly linked to reports of the following serious side effects:
- Acute pancreatitis (pancreas inflammation)
- Pancreatic cancer
- Thyroid cancer
- Heart failure
- Lactic acidosis
- Impaired liver function
- Kidney problems
Latest Diabetes News: Kombiglyze Linked to Increased Risk of Heart Failure
In the latest diabetes news, the FDA issued a Drug Safety Communication after the New England Journal of Medicine (NEJM) published a study linking saxagliptin (one of the main active ingredients in Kombiglyze XR) to a 27% increased risk of heart failure.
A study published by the New England Journal of Medicine in October 2013 first revealed that the use of Onglyza and Kombiglyze XR (saxagliptin) to treat type 2 diabetes is associated with significant cardiovascular risk.
In the study, 16,492 type-2 diabetes patients at risk of or with a history of cardiovascular events were studied for a two-year period. Half of these patients were administered Onglyza and Kombiglyze XR, and the other half were administered a placebo.
Patients administered Onglyza and Kombiglyze XR in the trial were found to have a 27 percent increased rate of hospitalization from heart failure and a higher risk of all-cause death than placebo patients.
FDA Investigates Kombiglyze XR Side Effects
The FDA took action after the NEJM study was published, issuing a February 2014 safety communication which stated they had requested clinical data from the manufacturers of saxagliptin in order to investigate the association between the drug and heart failure.
After the clinical data was submitted, the FDA’s Endocrinologic and Metabolic Advisory Committee convened to review the data. On April 14, 2015, the advisory panel voted 14-1 in favor of changing Kombiglyze XR and other diabetes medication labels to indicate an increased risk of heart failure, and mortality.
At the time, the panel did not recommend limiting distribution of the drugs or taking them off the market altogether.
What is Heart Failure and Its Symptoms?
Heart failure, distinct from heart attack or stroke, is a condition in which the heart cannot pump enough blood to meet the body’s needs. Heart failure occurs when the heart cannot fill with enough blood, or when it cannot pump the blood through the body with enough force.
Symptoms of heart failure may include:
- Fatigue
- Shortness of breath
- Irregular heart beat
- Persistent cough
- Swelling of the abdomen, legs or feet
- Chest pain
- Nausea
Kombiglyze XR Lawsuits
The FDA-recommended change to the Kombiglyze XR label has not yet been made and the type-2 diabetes treatment is currently being prescribed, sold, and used as-is by type-2 diabetes patients.
If you or a loved one has taken Onglyza or Kombiglyze XR and suffered from heart failure, you may qualify for a Kombiglyze XR lawsuit.
In general, Onglyza lawsuits are filed individually by each plaintiff and are not class actions.
Do YOU have a legal claim? Fill out the form on this page now for a free, immediate, and confidential case evaluation. The attorneys who work with Top Class Actions will contact you if you qualify to let you know if an individual Onglyza lawsuit or Ongylyza class action lawsuit is best for you. Hurry — statutes of limitations may apply.
ATTORNEY ADVERTISING
Top Class Actions is a Proud Member of the American Bar Association
LEGAL INFORMATION IS NOT LEGAL ADVICE
Top Class Actions Legal Statement
©2008 – 2024 Top Class Actions® LLC
Various Trademarks held by their respective owners
This website is not intended for viewing or usage by European Union citizens.
Get Help – It’s Free
Join a Free Onglyza Lawsuit Investigation
If you or a loved one were injured from Onglyza side effects such as heart failure, thyroid cancer or pancreatic cancer, you may have a legal claim. See if you qualify to pursue compensation and join a free Onglyza lawsuit investigation by submitting your information for a free case evaluation.
An attorney will contact you if you qualify to discuss the details of your potential case.
Oops! We could not locate your form.












